Cardiovascular Collapse, Cardiac Arrest, and Sudden
Cardiac Death
Overview
and Definitions
The
vast majority of naturally occurring sudden deaths are caused by cardiac
disorders. The magnitude of sudden cardiac death (SCD) as a public health
problem is highlighted by estimates that more than 300,000 deaths occur each
year in the
SCD
must be defined carefully. In the context of time, "sudden" is
defined, for most clinical and epidemiologic purposes, as 1 h or less between
the onset of the terminal clinical event, or an abrupt change in clinical
status, and death. An exception is unwitnessed deaths in which pathologists may
expand the definition of time to 24 h after the victim was last seen to be
alive and stable.
Because
of community-based interventions, victims may remain biologically alive for
days or even weeks after a cardiac arrest that has resulted in irreversible
central nervous system damage. Confusion in terms can be avoided by adhering
strictly to definitions of death, cardiac arrest, and cardiovascular collapse
(Table 39-1). Death is biologically, legally, and literally an absolute and irreversible
event. Death may be delayed in a survivor of cardiac arrest, but "survival
after sudden death" is an irrational term. Currently, the accepted
definition of SCD is natural death due to cardiac causes, heralded by
abrupt loss of consciousness within 1 h of the onset of acute symptoms,
in an individual who may have known preexisting heart disease but in
whom the time and mode of death are unexpected. When
biologic death of the cardiac arrest victim is delayed because of
interventions, the relevant pathophysiologic event remains the sudden and
unexpected cardiac arrest that leads ultimately to death, even though delayed
by artificial methods. The language used should reflect the fact that the index
event was a cardiac arrest and that death was due to its delayed consequences.
Table 39-1: Distinction Between Death, Cardiac
Arrest, and Cardiovascular Collapse
|
||||||||||||||||||||||||||||||||
Etiology, Initiating Events, and Clinical
Epidemiology
Clinical
and epidemiologic studies have identified populations at high risk for SCD. In
addition, a large body of pathologic data provides information on the
underlying structural abnormalities in victims of SCD, and studies of
clinical physiology have begun to identify a group of transient functional
factors that may convert a long-standing underlying structural abnormality
from a stable to an unstable state (Table 39-2). This information is developing
into an understanding of the causes and mechanisms of SCD.
Table 39-2: Cardiac Arrest and Sudden Cardiac
Death
|
Cardiac
disorders constitute the most common causes of sudden natural death. After
an initial peak incidence of sudden death between birth and 6 months of age
(the sudden infant death syndrome), the incidence of sudden death declines
sharply and remains low through childhood and adolescence. Among adolescents
and young adults, the incidence of SCD is approximately 1 per 100,000
population per year. The incidence begins to increase in adults over the age of
30 years, reaching a second peak in the age range of 45 to 75 years, when the
incidence approximates 1 to 2 per 1000 per year among the unselected adult
population. Increasing age within this range is a powerful risk factor for
sudden cardiac death, and the proportion of cardiac causes among all
sudden natural deaths increases dramatically with advancing years. From
Young
and middle-aged men and women have very different susceptibilities to SCD, but
the gender differences decrease with advancing age. In the 45- to 64-year-old
age group, the male SCD excess is nearly 7:1. It falls to 2:1 or less in the
65- to 74-year-old age group. The difference in risk for SCD parallels the
risks for other manifestations of coronary heart disease in men and women. As
the gender gap for manifestations of coronary heart disease closes in the
seventh and eighth decades of life, the excess risk of SCD in males also
narrows. Despite the lower incidence among younger women, coronary risk factors
such as cigarette smoking, diabetes, hyperlipidemia, and hypertension are
highly influential, and SCD remains an important clinical and epidemiologic
problem.
Hereditary factors contribute to the risk of SCD, but largely in a
nonspecific manner; they represent expressions of the hereditary predisposition
to coronary heart disease. A few specific syndromes, such as congenital long QT
interval syndromes (Chap. 230), right ventricular dysplasia, and the syndrome
of right bundle branch block and non-ischemic ST-segment elevations (Brugada
syndrome), are characterized by specific hereditary risk of SCD. There are also
recent data suggesting a familial predispositon to SCD as a specific pattern of
coronary heart disease expression.
The
major categories of structural causes of, and functional factors contributing
to, the SCD syndrome are listed in Table 39-2. Worldwide, and especially in
western cultures, coronary atherosclerotic heart disease is the most common
structural abnormality associated with SCD. Up to 80% of all SCDs in the United
States are due to the consequences of coronary atherosclerosis. The
cardiomyopathies (dilated and hypertrophic, collectively; Chap. 239) account
for another 10 to 15% of SCDs, and all the remaining diverse etiologies cause
only 5 to 10% of these events. Transient ischemia in the previously scarred or
hypertrophied heart, hemodynamic and fluid and electrolyte disturbances,
fluctuations in autonomic nervous system activity, and transient electrophysiologic
changes caused by drugs or other chemicals (e.g., proarrhythmia) have all been
implicated as mechanisms responsible for transition from electrophysiologic
stability to instability. In addition, reperfusion of ischemic myocardium may
cause transient electrophysiologic instability and arrhythmias.
Pathology
Data
from postmortem examinations of SCD victims parallel the clinical observations
on the prevalence of coronary heart disease as the major structural etiologic
factor. More than 80% of SCD victims have pathologic findings of coronary heart
disease. The pathologic description often includes a combination of
long-standing, extensive atherosclerosis of the epicardial coronary arteries
and acute active coronary lesions, which include a combination of fissured or
ruptured plaques, platelet aggregates, hemorrhage, and thombosis. In one study,
chronic coronary atherosclerosis involving two or more major vessels with ![]() 75%
stenosis was observed in 75% of the victims. In another study, atherosclerotic
plaque fissuring, platelet aggregates, and/or acute thrombosis were observed in
95 of 100 individuals who had pathologic studies after SCD.
75%
stenosis was observed in 75% of the victims. In another study, atherosclerotic
plaque fissuring, platelet aggregates, and/or acute thrombosis were observed in
95 of 100 individuals who had pathologic studies after SCD.
As
many as 70 to 75% of males who die suddenly have prior myocardial infarctions
(MIs), but only 20 to 30% have recent acute MIs. A high incidence of left
ventricular (LV) hypertrophy coexists with prior MIs.
Clinical Definition of Forms of Cardiovascular
Collapse (Table
39-1)
Cardiovascular collapse is a general term connoting loss of effective
blood flow due to acute dysfunction of the heart and/or peripheral vasculature.
Cardiovascular collapse may be caused by vasodepressor syncope (vasovagal
syncope, postural hypotension with syncope, neurocardiogenic syncope-Chap. 21),
a transient severe bradycardia, or cardiac arrest. The latter is distinguished
from the transient forms of cardiovascular collapse in that it usually requires
an intervention to achieve resuscitation. In contrast, vasodepressor syncope
and many primary bradyarrhythmic syncopal events are transient and
non-life-threatening, with spontaneous return of consciousness.
The
most common electrical mechanism for true cardiac arrest is ventricular
fibrillation (VF), which is responsible for 65 to 80% of cardiac arrests. Severe
persistent bradyarrhythmias, asystole, and pulseless electrical activity (an
organized electrical activity without mechanical response, formerly called
electomechanical dissociation) cause another 20 to 30%. Sustained ventricular
tachycardia (VT) with hypotension is a less common cause. Acute low cardiac
output states, having precipitous onset, also may present clinically as a
cardiac arrest. The causes include massive acute pulmonary emboli, internal
blood loss from ruptured aortic aneurysm, intense anaphylaxis, cardiac rupture
after myocardial infarction, and unexpected fatal arrhythmia due to electrolyte
disturbances.
Clinical Characteristics of Cardiac Arrest
Prodrome, Onset, Arrest, Death
SCD
may be presaged by days, weeks, or months of increasing angina, dyspnea,
palpitations, easy fatigability, and other nonspecific complaints. However,
these prodromal complaints are generally predictive of any major cardiac
event; they are not specific for predicting SCD.
The
onset of the terminal event, leading to cardiac arrest, is defined as an
acute change in cardiovascular status preceding cardiac arrest by up to 1 h.
When the onset is instantaneous or abrupt, the probability that the arrest is
cardiac in origin is >95%. Continuous ECG recordings, fortuitously obtained
at the onset of a cardiac arrest, commonly demonstrate changes in cardiac
electrical activity in the minutes or hours before the event. There is a
tendency for the heart rate to increase and for advanced grades of premature
ventricular contractions (PVCs) to evolve. Most cardiac arrests that are caused
by VF begin with a run of sustained or nonsustained VT, which then degenerates
into VF.
Sudden
unexpected loss of effective circulation may be separated into "arrhythmic
events" and "circulatory failure." Arrhythmic events are
characterized by a high likelihood of patients being awake and active
immediately prior to the event, are dominated by VF as the electrical
mechanism, and have a short duration of terminal illness (<1 h). In contrast,
circulatory failure deaths occur in patients who are inactive or comatose, have
a higher incidence of asystole than VF, have a tendency to a longer duration of
terminal illness, and are dominated by noncardiac events preceding the terminal
illness.
The
onset of cardiac arrest may be characterized by typical symptoms of an acute
cardiac event, such as prolonged angina or the pain of onset of MI, acute
dyspnea or orthopnea, or the sudden onset of palpitations, sustained
tachycardia, or light-headedness. However, in many patients, the onset is
precipitous, with minimal forewarning.
Cardiac arrest is, by definition, abrupt. Mentation may be
impaired in patients with sustained VT during the onset of the terminal event.
However, complete loss of consciousness is a sine qua non in cardiac
arrest. Although rare spontaneous reversions occur, it is usual that cardiac
arrest progresses to death within minutes (i.e., SCD has occurred) if active
interventions are not undertaken promptly.
The
probability of achieving successful resuscitation from cardiac arrest is
related to the interval from onset to institution of resuscitative efforts, the
setting in which the event occurs, the mechanism (VF, VT, pulseless electrical
activity, asystole), and the clinical status of the patient prior to the
cardiac arrest. Those settings in which it is possible to institute prompt
cardiopulmonary resuscitation (CPR) provide a better chance of a successful
outcome. However, the outcome in intensive care units and other in-hospital
environments is heavily influenced by the patient's preceding clinical status.
The immediate outcome is good for cardiac arrest occurring in the intensive
care unit in the presence of an acute cardiac event or transient metabolic
disturbance, but the outcome for patients with far-advanced chronic cardiac
disease or advanced noncardiac diseases (e.g., renal failure, pneumonia,
sepsis, diabetes, cancer) is not much more successful in hospital than in the
out-of-hospital setting.
The success rate for initial resuscitation and
survival to hospital discharge after an out-of-hospital cardiac arrest depends
in part on the mechanism of the event. When the mechanism is VT, the outcome is
best; VF is the next most successful; and asystole and pulseless electrical
activity generate dismal outcome statistics (Fig. 39-1). Advanced age also
influences adversely the chances of successful resuscitation.
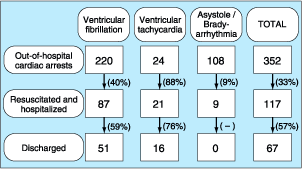
Figure 39-1: Initial electrophysiologic mechanisms
recorded during out-of-hospital cardiac arrest. The figures highlighted by the
boxes indicate the number of patients in each of three mechanism categories
(ventricular fibrillation, ventricular tachycardia, and bradyarrhythmia/asystole).
In each category, the data indicate the number of prehospital cardiac arrests (top),
the number of patients successfully resuscitated in the field and transferred
to the hospital alive (middle), and the number of patients who survived
to be discharged from hospital (bottom). The percentages in parentheses
indicate survivals between each level of care for each category.
Progression to biologic death is a function of the
mechanism of cardiac arrest and the length of the delay before interventions.
VF or asystole without CPR within the first 4 to 6 min has a poor outcome, and
there are few survivors among patients who had no life support activities for
the first 8 min after onset. Outcome statistics are improved by lay bystander
intervention (basic life support-see below) prior to definitive interventions
(advanced life support-defibrillation) and even more by early defibrillation.
In regard to the latter, the notion that deployment of automatic external
defibrillators in communities (e.g., police vehicles, large buildings,
stadiums, etc.) will result in improved survival is currently being evaluated.
Death
during the hospitalization after a successfully resuscitated cardiac arrest
relates closely to the severity of central nervous system injury. Anoxic
encephalopathy and infections subsequent to prolonged respirator dependence
account for 60% of the deaths. Another 30% occur as a consequence of low
cardiac output states that fail to respond to interventions. Recurrent
arrhythmias are the least common cause of death, accounting for only 10% of
in-hospital deaths.
In
the setting of acute MI, it is important to distinguish between primary and
secondary cardiac arrests. Primary cardiac arrests refer to those that
occur in the absence of hemodynamic instability, and secondary cardiac
arrests are those that occur in patients in whom abnormal hemodynamics dominate
the clinical picture before cardiac arrest. The success rate for immediate
resuscitation in primary cardiac arrest during acute MI in a monitored setting
should approach 100%. In contrast, as many as 70% of patients with secondary
cardiac arrest succumb immediately or during the same hospitalization.
Identification of Patients at Risk for Sudden
Cardiac Death
Primary prevention of cardiac arrest depends on
the ability to identify individual patients at high risk. One must view the
problem in the context of the total number of events and the population pools
from which they are derived. The annual incidence of SCD among an unselected
adult population is 1 to 2 per 1000 population (Fig. 39-2A), largely
reflecting the prevalence of those coronary heart disease patients among whom
SCD is the first clinically recognized manifestation (20 to 25% of first
coronary events are SCD). The incidence (percent per year) increases
progressively with the addition of identified coronary risk factors to
populations free of prior coronary events. The most powerful factors are age,
elevated blood pressure, LV hypertrophy, cigarette smoking, elevated serum
cholesterol level, obesity, and nonspecific electrocardiographic abnormalities.
These coronary risk factors are not specific for SCD but rather represent
increasing risk for all coronary deaths. The proportion of coronary deaths that
are sudden remains at approximately 50% in all risk categories. Despite the
marked relative increased risk of SCD with addition of multiple risk
factors (from 1 to 2 per 1000 population per year in an unselected population
to as much as 50 to 60 per 1000 in subgroups having multiple risk factors for
coronary artery disease), the absolute incidence remains relatively low
when viewed as the relationship between the number of individuals who have a
preventive intervention and the number of events that can be prevented.
Specifically, a 50% reduction in annual SCD risk would be a huge relative
decrease but would require an intervention in up to 200 unselected individuals
to prevent one sudden death. These figures highlight the importance of primary
prevention of coronary heart disease. Control of coronary risk factors may be
the only practical method to prevent SCD in major segments of the population,
because of the paradox that the majority of events occur in the large
unselected subgroups rather than in the specific high-risk subgroups (compare
"Events/Year" with "Percent/Year" in Fig. 39-2A).
Under most conditions of higher level of risk, particularly those indexed to a
recent major cardiovascular event (e.g., MI, recent onset of heart failure,
survival after out-of-hospital cardiac arrest), the highest risk of sudden
death occurs within the initial 6 to 18 months and then decreases toward
baseline risk of the underlying disease (Fig. 39-2B). Accordingly,
preventive interventions are most likely to be effective when initiated early.
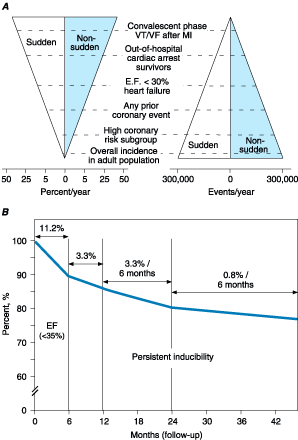
Figure 39-2: A. Incidence of sudden
and nonsudden cardiac deaths in population subgroups, and the relation of total
number of events per year to incidence figures. Approximations of subgroup
incidence figures, and the related population pool from which they are derived,
are presented. Approximately 50% of all cardiac deaths are sudden and
unexpected. The incidence triangle on the left ("Percent/Year")
indicates the approximate percentage of sudden and nonsudden deaths in each of
the population subgroups indicated, ranging from the lowest percentage in
unselected adult populations (0.1 to 2% per year) to the highest percentage in
patients with VT or VF during convalescence after an MI (approximately 50% per
year). The triangle on the right indicates the total number of events per year
in each of these groups, to reflect incidence in context with the size of the
population subgroups. The highest risk categories identify the smallest number
of total annual events, and the lowest incidence category accounts for the
largest number of events per year. (EF, ejection fraction; VT, ventricular
tachycardia; VF, ventricular fibrillation; MI, myocardial infarction.) B.
Time dependence of risk among survivors of out-of-hospital cardiac arrest.
Recurrence risk is highest in the first 6 months of the index event. Survival
is expressed as a percentage. High risk is best predicted initially by an
ejection fraction ![]() 35%
during the first 6 months, and subsequently persistent inducibility of VT
during electrophysiologic testing becomes an added major risk. n = 101
at t = 0.
35%
during the first 6 months, and subsequently persistent inducibility of VT
during electrophysiologic testing becomes an added major risk. n = 101
at t = 0.
For
patients with acute or prior clinical manifestations of coronary heart disease,
high-risk subgroups having a much higher ratio of SCD risk to population base can
be identified. The acute, convalescent, and chronic phases of MI provide large
population subsets with more highly focused risk (Chap. 243). The potential
risk of cardiac arrest from the onset through the first 72 h after acute MI
(the acute phase) may be as high as 15 to 20%. The highest risk of SCD in
relation to MI is found in the subgroup that has experienced sustained VT or VF
during the convalescent phase (3 days to 8 weeks) after MI. A greater than 50%
mortality in 6 to 12 months has been observed among these patients, when
managed with conservative medical therapy, and at least 50% of the deaths are
sudden. Aggressive intervention techniques may reduce this incidence.
After
the acute phase of MI, long-term risk for total mortality and SCD are predicted
by a number of factors. The most important for both SCD and non-SCD is the
extent of myocardial damage sustained during the acute event. This is measured
by the degree of reduction in the ejection fraction (EF), functional capacity,
and/or the occurrence of heart failure. Increasing frequency of
postinfarction PVCs, with a plateau above the range of 10 to 30 PVCs per hour
on 24-h ambulatory monitor recordings, also indicates increased risk, but
advanced forms (salvos, nonsustained VT) may be more powerful
predictors. PVCs interact strongly with decreased left ventricular EF. The
combination of frequent PVCs, salvos or nonsustained VT, and an EF ![]() 35%
identifies patients who have an annual risk of greater than 20%. The risk falls
off sharply with decreasing PVC frequency and the absence of advanced forms, as
well as with higher EF. Despite the risk implications of postinfarction PVCs,
improved outcome as a result of PVC suppression has not been demonstrated
(Chap. 230).
35%
identifies patients who have an annual risk of greater than 20%. The risk falls
off sharply with decreasing PVC frequency and the absence of advanced forms, as
well as with higher EF. Despite the risk implications of postinfarction PVCs,
improved outcome as a result of PVC suppression has not been demonstrated
(Chap. 230).
The
extent of underlying disease due to any cause and/or prior clinical expression
of risk of SCD (i.e., survival after out-of-hospital cardiac arrest not
associated with acute MI) identify patients at very high risk for subsequent
(recurrent) cardiac arrest. Survival after out-of-hospital cardiac arrest
predicts up to a 30% 1-year recurrent cardiac arrest rate in the absence of
specific interventions (see below).
A
general rule is that the risk of SCD is approximately one-half the total
cardiovascular mortality rate. As shown in Fig. 39-2A, the very high
risk subgroups provide more focused population fractions
("Percent/Year") for predicting cardiac arrest or SCD; but the impact
on the overall population, indicated by the absolute number of preventable
events ("Events/Year"), is considerably smaller. The requirements for
achieving a major population impact are effective prevention of the underlying
diseases and/or new epidemiologic probes that will allow better resolution of
subgroups within large general populations.
![]() Treatment
Treatment
The
individual who collapses suddenly is managed in four stages: (1) the initial
response and basic life support; (2) advanced life support; (3)
postresuscitation care; and (4) long-term management. The initial response and
basic life support can be carried out by physicians, nurses, paramedical
personnel, and trained lay persons. There is a requirement for increasingly
specialized skills as the patient moves through the stages of advanced life
support, postresuscitation care, and long-term management.
Initial Response and Basic Life Support
The
initial response will confirm whether a sudden collapse is indeed due to a
cardiac arrest. Observations for respiratory movements, skin color, and the presence
or absence of pulses in the carotid or femoral arteries will promptly determine
whether a life-threatening cardiac arrest has occurred. As soon as a cardiac
arrest is suspected or confirmed, contacting an emergency rescue system (e.g.,
911) should be the immediate priority.
Agonal
respiratory movements may persist for a short time after the onset of cardiac
arrest, but it is important to observe for severe stridor with a persistent
pulse as a clue to aspiration of a foreign body or food. If this is suspected,
a Heimlich maneuver (see below) may dislodge the obstructing body. A precordial
blow, or "thump," delivered firmly by the clenched fist to the
junction of the middle and lower third of the sternum may occasionally revert
VT or VF, but there is concern about converting VT to VF. Therefore, it
has been recommended to use precordial thumps as an advanced life support
technique when monitoring and defibrillation are available. This conservative
application of the technique remains controversial.
The
third action during the initial response is to clear the airway. The head is
tilted back and chin lifted so that the oropharynx can be explored to clear the
airway. Dentures or foreign bodies are removed, and the Heimlich maneuver is
performed if there is reason to suspect that a foreign body is lodged in the
oropharynx. If respiratory arrest precipitating cardiac arrest is suspected, a
second precordial thump is delivered after the airway is cleared.
Basic
life support, more popularly known as CPR, is intended to maintain organ
perfusion until definitive interventions can be instituted. The elements of CPR
are the maintenance of ventilation of the lungs and compression of the chest.
Mouth-to-mouth respiration may be used if no specific rescue equipment is immediately
available (e.g., plastic oropharyngeal airways, esophageal obturators, masked
Ambu bag). Conventional ventilation techniques during CPR require the lungs to
be inflated 10 to 12 times per minute, i.e., once every fifth chest compression
when two persons are performing the resuscitation and twice in succession every
15 chest compressions when one person is carrying out both ventilation and
chest wall compression.
Chest
compression is based on the assumption that cardiac compression allows the heart
to maintain a pump function by sequential filling and emptying of its chambers,
with competent valves maintaining forward direction of flow. The palm of one
hand is placed over the lower sternum, with the heel of the other resting on
the dorsum of the lower hand. The sternum is depressed, with the arms remaining
straight, at a rate of approximately 80 to 100 per minute. Sufficient force is
applied to depress the sternum 3 to 5 cm, and relaxation is abrupt.
Advanced Life Support
Advanced
life support is intended to achieve adequate ventilation, control cardiac
arrhythmias, stabilize blood pressure and cardiac output, and restore organ
perfusion. The activities carried out to achieve these goals include (1)
intubation with an endotracheal tube, (2) defibrillation/cardioversion and/or
pacing, and (3) insertion of an intravenous line. Ventilation with O2
(room air if O2 is not immediately available) may promptly reverse
hypoxemia and acidosis. The speed with which defibrillation/cardioversion is
carried out is an important element for successful resuscitation. When
possible, immediate defibrillation should precede intubation and insertion of
an intravenous line; CPR should be carried out while the defibrillator is being
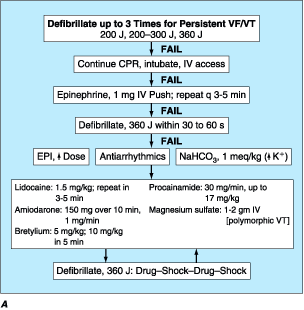
charged. As soon as a diagnosis of VT or VF is obtained, a 200-J shock should
be delivered. Additional shocks at higher energies, up to a maximum of 360 J,
are tried if the initial shock does not successfully abolish VT or VF.
Epinephrine, 1 mg intravenously, is given after failed defibrillation, and
attempts to defibrillate are repeated. The dose of epinephrine may be repeated
after intervals of 3 to 5 min (see Fig. 39-3A).
|
|
|
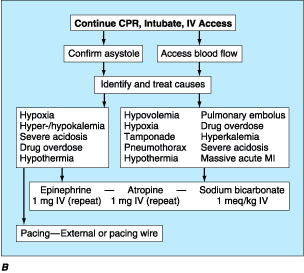
Figure 39-3: Management of cardiac arrest. A.
The algorithm of ventricular fibrillation or hypotensive ventricular tachycardia
begins with defibrillation attempts. If that fails, it is followed by
epinephrine and then antiarrhythmic drugs. See text for details. B. The
algorithms for bradyarrhythmia/asystole (left) or pulseless electrical
activity (right) is dominated first by continued life support and a
search for reversible causes. Subsequent therapy is nonspecific and accompanied
by a low success rate. See text for details.
If
the patient is less than fully conscious upon reversion, or if two or three
attempts fail, prompt intubation, ventilation, and arterial blood gas analysis
should be carried out. Intravenous NaHCO3, which was formerly used
in large quantities, is no longer considered routinely necessary and may be
dangerous in larger quantities. However, the patient who is persistently
acidotic after successful defibrillation and intubation should be given 1
meq/kg NaHCO3 initially and an additional 50% of the dose repeated
every 10 to 15 min.
After
initial unsuccessful defibrillation attempts, or with persistent electrical
instability, a bolus of 1 mg/kg lidocaine is given intravenously (Chap. 243),
and the dose is repeated in 2 min in those patients who have persistent
ventricular arrhythmias or remain in VF. This is followed by a continuous
infusion at a rate of 1 to 4 mg/min. If lidocaine fails to provide control,
other antiarrhythmic therapies should be tried. For persistent, hemodynamically
unstable ventricular arrhythmias, intravenous amiodarone has emerged as the
treatment of choice (150 mg over 10 min, followed by 1 mg/min for up to 6 h,
and 0.5 mg/min thereafter) (Fig. 39-3A). Intravenous procainamide
(loading infusion of 100 mg/5 min to a total dose of 500 to 800 mg, followed by
continuous infusion at 2 to 5 mg/min) may be tried for persisting, hemodynamically
stable arrhythmias; or bretylium tosylate (loading dose 5 to 10 mg/kg in 5 min;
maintenance dose 0.5 to 2 mg/min) may be tried as an alternative for unstable
arrhythmias. Intravenous calcium gluconate is no longer considered safe or
necessary for routine administration. It is used only in patients in whom acute
hyperkalemia is known to be the triggering event for resistant VF, in the
presence of known hypocalcemia, or in patients who have received toxic doses of
calcium channel antagonists.
Cardiac
arrest secondary to bradyarrhythmias or asystole is managed differently (Fig.
39-3B). The patient is promptly intubated, CPR is continued, and an
attempt is made to control hypoxemia and acidosis. Epinephrine and/or atropine are
given intravenously or by an intracardiac route. External pacing devices are
now available to attempt to establish a regular rhythm, but the prognosis is
generally very poor in this form of cardiac arrest, even with successful
electrical pacing. Pulseless electrical activity (PEA) is treated similarly to
bradyarrhythmias, but its outcome is also dismal. The one exception is
bradyarrhythmic/asystolic cardiac arrest secondary to airway obstruction. This
form of cardiac arrest may respond promptly to removal of foreign bodies by the
Heimlich maneuver or, in hospitalized patients, by intubation and suctioning of
obstructing secretions in the airway.
Postresuscitation Care
This
phase of management is determined by the clinical setting of the cardiac
arrest. Primary VF in acute MI (Chap. 243) is generally very responsive
to life-support techniques and easily controlled after the initial event.
Patients are maintained on a lidocaine infusion at the rate of 2 to 4 mg/min
for 24 to 72 h after the event. In the in-hospital setting, respirator support
is usually not necessary or is needed for only a short time, and hemodynamics
stabilize promptly after defibrillation or cardioversion. In secondary
VF in acute MI (those events in which hemodynamic abnormalities predispose to
the potentially fatal arrhythmia), resuscitative efforts are less often
successful, and in those patients who are successfully resuscitated, the
recurrence rate is high. The clinical picture and outcome are dominated by
hemodynamic instability and the ability to control hemodynamic dysfunction.
Bradyarrhythmias, asystole, and pulseless electrical activity are commonly
secondary events in hemodynamically unstable patients.
The
outcome after in-hospital cardiac arrest associated with non-cardiac
diseases is poor, and in the few successfully resuscitated patients, the
postresuscitation course is dominated by the nature of the underlying disease.
Patients with cancer, renal failure, acute central nervous system disease, and
uncontrolled infections, as a group, have a survival rate of less than 10%
after in-hospital cardiac arrest. Some major exceptions are patients with
transient airway obstruction, electrolyte disturbances, proarrhythmic effects
of drugs, and severe metabolic abnormalities, most of whom may have an
excellent chance of survival if they can be resuscitated promptly and
maintained while the transient abnormalities are being corrected.
Long-Term Management after Survival of
Out-of-Hospital Cardiac Arrest
Patients
who do not suffer irreversible injury of the central nervous system and who
achieve hemodynamic stability should have extensive diagnostic and therapeutic
testing to guide long-term management. This aggressive approach is driven by
the fact that statistics from the 1970s indicated survival after
out-of-hospital cardiac arrest was followed by a 30% recurrent cardiac arrest
rate at 1 year, 45% at 2 years, and a total mortality rate of almost 60% at 2
years. Historical comparisons suggest that these dismal statistics may be
significantly improved by newer interventions, but the magnitude of the
improvement is unknown because of the lack of concurrently controlled
intervention studies.
Among
those patients in whom an acute transmural MI is the cause of out-of-hospital
cardiac arrest, the management is the same as in any other patient who suffers
cardiac arrest during the acute phase of a documented MI (Chap. 243). For
almost all other categories of patients, however, extensive diagnostic studies
are carried out to determine etiology, functional impairment, and
electrophysiologic instability as guides to future management. In general,
patients who have out-of-hospital cardiac arrest due to chronic ischemic heart
disease, without an acute MI, are evaluated to determine whether transient
ischemia or chronic electrophysiologic instability was the more likely cause of
the event. If there is reason to suspect an ischemic mechanism, coronary
revascularization by angioplasty or bypass surgery, plus drugs (most commonly
beta blockers), are used to reduce ischemic burden.
Electrophysiologic
instability has been identified by the use of programmed electrical stimulation
to determine whether sustained VT or VF can be induced (Chap. 230). If so, this
information can be used as a baseline against which to evaluate drug efficacy
for prevention of inducibility. The rationale for this approach is the
assumption that suppression of inducibility predicts long-term benefit by the
drug that achieves such suppression. For patients for whom successful drug
therapy could not be identified by this technique, insertion of an implantable
cardioverter-defibrillator (ICD), antiarrhythmic surgery (e.g., coronary bypass
surgery, aneurysmectomy, cryoablation), or empiric amiodarone therapy have been
recommended (Chap. 230). Primary surgical success, defined as surviving the
procedure and reverting to a noninducible status without drug therapy, is
better than 90% when patients are selected for ability to be mapped in the
operating room. However, only a small fraction of patients meet the criteria.
In addition, VT/VF cannot be induced in a number of survivors of cardiac
arrest (30 to 50%), and inducible arrhythmias can be suppressed by drugs in no
more than 20 to 30% of those whose arrhythmias can be induced. Because of these
limitations of drug therapy and surgical approaches, ICD therapy has evolved
into the most commonly used strategy for cardiac arrest survivors. ICDs have
long been recognized to have very good success rates for sensing and reverting
life-threatening arrhythmias, but improvement in long-term total survival
outcomes remained lacking until a number of studies solidified the benefit of
ICD therapy for specific subgroups. After empiric amiodarone therapy had been
suggested to be as good as, or better than, conventional antiarrhythmic drug
therapy for survivors of cardiac arrest, ICDs were demonstrated to be superior
to amiodarone. Moreover, ICDs were also found to be superior for high risk
patients with VT after myocardial infarction.