Tuberculosis
Definition
Tuberculosis, one of the oldest diseases known to affect humans, is caused by bacteria belonging to the Mycobacterium tuberculosis complex. The disease usually affects the lungs, although in up to one-third of cases other organs are involved. If properly treated, tuberculosis caused by drug-susceptible strains is curable in virtually all cases. If untreated, the disease may be fatal within 5 years in more than half of cases. Transmission usually takes place through the airborne spread of droplet nuclei produced by patients with infectious pulmonary tuberculosis.
Etiologic Agent
Mycobacteria belong to the family Mycobacteriaceae and the order Actinomycetales. Of the pathogenic species belonging to the M. tuberculosis complex, the most frequent and important agent of human disease is M. tuberculosis itself. The complex includes M. bovis (the bovine tubercle bacillus, once an important cause of tuberculosis transmitted by unpasteurized milk and currently the cause of a small percentage of cases in developing countries), M. africanum (isolated in a small proportion of cases in West and Central Africa), and M. microti (the "vole" bacillus, a closely related but rarely encountered organism).
M. tuberculosis is a rod-shaped, non-spore-forming, thin aerobic bacterium measuring about 0.5 ![]() m by 3
m by 3 ![]() m. Mycobacteria, including M. tuberculosis, do not stain readily and are often neutral on Gram's staining. However, once stained, the bacilli cannot be decolorized by acid alcohol, a characteristic justifying their classification as acid-fast bacilli (AFB). Acid fastness is due mainly to the organisms' high content of mycolic acids, long-chain cross-linked fatty acids, and other cell-wall lipids. Microorganisms other than mycobacteria that display some acid fastness include species of Nocardia and Rhodococcus, Legionella micdadei, and the protozoa Isospora and Cryptosporidium. In the mycobacterial cell wall, lipids (e.g., mycolic acids) are linked to underlying arabinogalactan and peptidoglycan. This structure is responsible for the very low permeability of the cell wall and thus for the ineffectiveness of most antibiotics against the organism. Another molecule in the mycobacterial cell wall, lipoarabinomannan, is involved in the pathogen-host interaction and facilitates the survival of M. tuberculosis within macrophages. The several proteins characteristic of M. tuberculosis include those in purified protein derivative (PPD) tuberculin, a precipitate of non-species-specific molecules obtained from filtrates of heat-sterilized, concentrated broth cultures. The complete genome sequence of M. tuberculosis comprises about 4000 genes and has a high guanine-plus-cytosine content. A large proportion of the genes are devoted to the production of enzymes involved in lipogenesis and lipolysis and of glycine-rich proteins that are probably responsible for antigenic variations.
m. Mycobacteria, including M. tuberculosis, do not stain readily and are often neutral on Gram's staining. However, once stained, the bacilli cannot be decolorized by acid alcohol, a characteristic justifying their classification as acid-fast bacilli (AFB). Acid fastness is due mainly to the organisms' high content of mycolic acids, long-chain cross-linked fatty acids, and other cell-wall lipids. Microorganisms other than mycobacteria that display some acid fastness include species of Nocardia and Rhodococcus, Legionella micdadei, and the protozoa Isospora and Cryptosporidium. In the mycobacterial cell wall, lipids (e.g., mycolic acids) are linked to underlying arabinogalactan and peptidoglycan. This structure is responsible for the very low permeability of the cell wall and thus for the ineffectiveness of most antibiotics against the organism. Another molecule in the mycobacterial cell wall, lipoarabinomannan, is involved in the pathogen-host interaction and facilitates the survival of M. tuberculosis within macrophages. The several proteins characteristic of M. tuberculosis include those in purified protein derivative (PPD) tuberculin, a precipitate of non-species-specific molecules obtained from filtrates of heat-sterilized, concentrated broth cultures. The complete genome sequence of M. tuberculosis comprises about 4000 genes and has a high guanine-plus-cytosine content. A large proportion of the genes are devoted to the production of enzymes involved in lipogenesis and lipolysis and of glycine-rich proteins that are probably responsible for antigenic variations.
Epidemiology
Between 3.5 and 4 million new cases of tuberculosis-all forms (pulmonary and extrapulmonary), 90% of them from developing countries-were reported annually to the World Health Organization (WHO) in the late 1990s. However, because of a low level of case detection and incomplete notifications in many national programs, reported cases represent only a fraction of the total. It is estimated that 8 million new cases of tuberculosis occurred worldwide in 1997, 95% of them in developing countries of Asia (5 million), Africa (1.6 million), the Middle East (0.6 million), and Latin America (0.4 million). It is also estimated that nearly 2 million deaths from tuberculosis occurred in 1997, 98% of them in developing countries.
Beginning in the mid-1980s in many industrialized countries, the number of tuberculosis case notifications, which had been falling steadily, stabilized or even began to increase. This phenomenon was first noted in the United States but was soon observed in many European countries as well. A number of factors were implicated in the resurgence of tuberculosis in the United States in 1986 through 1992-most notably, immigration from countries with a high prevalence of tuberculosis; infection with HIV; emergence of multidrug-resistant (MDR) tuberculosis due to strains resistant at least to isoniazid and rifampin; and social problems such as poverty, homelessness, and drug abuse. In some areas (e.g., New York City), deterioration in the public health system and dismantling of tuberculosis management services also contributed to the worsening situation.With the implementation of stronger control programs, cases began to decrease in 1993. In 1998, 18,361 cases of tuberculosis (6.8 per 100,000 population) were reported to the U.S. Centers for Disease Control and Prevention (CDC)-a 31% decrease from the 1992 peak.
In the United States, tuberculosis is uncommon among young adults of European descent, who have only rarely been exposed to M. tuberculosis infection during recent decades. In contrast, because of a high risk in the past, the prevalence of M. tuberculosis infection is relatively high among elderly Caucasians, who remain at increased risk of developing active tuberculosis. Tuberculosis in the United States is also a disease of young adult members of the HIV-infected, immigrant, and disadvantaged/marginalized populations. Similarly, in Europe, tuberculosis has reemerged as an important public health problem, mainly as a result of cases among immigrants from high-prevalence countries.
In developing countries of Africa and Asia, tuberculosis trends over the past several decades are not entirely clear. However, in sub-Saharan African countries with reliable reporting systems, the recent spread of the HIV epidemic has been accompanied by doubling or tripling of the number of reported cases of tuberculosis during a period as short as 10 years. The growing number of young adults with M. tuberculosis infection has fueled the rates of active tuberculosis in many developing countries. Without greater control efforts, the annual incident cases of tuberculosis globally may increase by 40% between now and 2020.
From Exposure to Infection
M. tuberculosis is most commonly transmitted from a patient with infectious pulmonary tuberculosis to other persons by droplet nuclei, which are aerosolized by coughing, sneezing, or speaking. The tiny droplets dry rapidly; the smallest (<5 to 10 ![]() m in diameter) may remain suspended in the air for several hours and may gain direct access to the terminal air passages when inhaled. There may be as many as 3000 infectious nuclei per cough. In the past, a frequent source of infection was raw milk containing M. bovis from tuberculous cows. Other routes of transmission of tubercle bacilli, such as through the skin or the placenta, are uncommon and of no epidemiologic significance.
m in diameter) may remain suspended in the air for several hours and may gain direct access to the terminal air passages when inhaled. There may be as many as 3000 infectious nuclei per cough. In the past, a frequent source of infection was raw milk containing M. bovis from tuberculous cows. Other routes of transmission of tubercle bacilli, such as through the skin or the placenta, are uncommon and of no epidemiologic significance.
The probability of contact with a case of tuberculosis, the intimacy and duration of that contact, the degree of infectiousness of the case, and the shared environment of the contact are all important determinants of transmission. Several studies of close contacts have clearly demonstrated that tuberculosis patients whose sputum contains AFB visible by microscopy play the greatest role in the spread of infection. These patients often have cavitary pulmonary disease or tuberculosis of the respiratory tract (endobronchial or laryngeal tuberculosis) and produce sputa containing as many as 105 AFB/mL. Patients with sputum smear-negative/culture-positive tuberculosis are less infectious, and those with culture-negative pulmonary disease and extrapulmonary tuberculosis are essentially noninfectious. Crowding in poorly ventilated rooms is one of the most important factors in the transmission of tubercle bacilli, since it increases the intensity of contact with a case.
In short, the risk of acquiring M. tuberculosis infection is determined mainly by exogenous factors. Because of delays in seeking care and in diagnosis, it is estimated that up to 20 contacts will usually be infected by each AFB-positive case before detection in high-prevalence countries.
From Infection to Disease
Unlike the risk of acquiring infection with M. tuberculosis, the risk of developing disease after being infected depends largely on endogenous factors, such as the individual's innate susceptibility to disease and level of function of cell-mediated immunity. Clinical illness directly following infection is classified as primary tuberculosis and is common among children up to 4 years of age. Although this form may be severe and disseminated, it is usually not transmissible. When infection is acquired later in life, the chance is greater that the immune system will contain it, at least temporarily. The majority of infected individuals who ultimately develop tuberculosis do so within the first year or two after infection. Dormant bacilli, however, may persist for years before being reactivated to produce secondary (or postprimary) tuberculosis, which is often infectious. Overall, it is estimated that about 10% of infected persons will eventually develop active tuberculosis. Reinfection of a previously infected individual, which is probably common in areas with high rates of tuberculosis transmission, may also favor the development of disease. Molecular typing and comparison of strains of M. tuberculosis have suggested that up to one-third of cases of active tuberculosis in U.S. inner-city communities are due to recent transmission rather than to reactivation of latent infection.
Age is an important determinant of the risk of disease after infection. Among infected persons, the incidence of tuberculosis is highest during late adolescence and early adulthood; the reasons are unclear. The incidence among women peaks at 25 to 34 years of age. In this age group rates among women are usually higher than those among men, while at older ages the opposite is true. The risk may increase in the elderly, possibly because of waning immunity and comorbidity.
A variety of diseases favor the development of active tuberculosis. The most potent risk factor for tuberculosis among infected individuals is clearly HIV co-infection, which suppresses cellular immunity. The risk that latent M. tuberculosis infection will proceed to active disease is directly related to the patient's degree of immunosuppression. In a study of HIV-infected, PPD-positive persons, this risk varied from 2.6 to 13.3 cases per 100 person-years and depended upon the CD4+ cell count. The risk of developing tuberculosis is significantly greater among HIV-infected than among HIV-uninfected hosts. Other conditions known to increase the risk of active tuberculosis among persons infected with tubercle bacilli include silicosis; lymphoma, leukemia, and other malignant neoplasms; hemophilia; chronic renal failure and hemodialysis; insulin-dependent diabetes mellitus; immunosuppressive treatment, including that administered for solid-organ transplantation; and conditions associated with malnutrition, such as gastrectomy and jejunoileal bypass surgery. Finally, the presence of old, self-healed, fibrotic tuberculous lesions constitutes a serious risk of active disease.
Natural History of Disease
Studies conducted in various countries before the advent of chemotherapy clearly showed that untreated tuberculosis is often fatal. About one-third of patients died within 1 year after diagnosis, and one-half within 5 years. Five-year mortality among sputum smear-positive cases was 65%. Of the survivors at 5 years, about 60% had undergone spontaneous remission, while the remainder were still excreting tubercle bacilli.
The introduction of effective chemotherapy has markedly affected the natural history of tuberculosis. With proper treatment, patients have a high chance of being cured. However, improper use of antituberculosis drugs, while reducing mortality, may also result in large numbers of chronic infectious cases, often with drug-resistant bacilli.
Pathogenesis and Immunity
The interaction of M. tuberculosis with the human host begins when droplet nuclei containing microorganisms from infectious patients are inhaled. While the majority of inhaled bacilli are trapped in the upper airways and expelled by ciliated mucosal cells, a fraction (usually fewer than 10%) reach the alveoli. There, nonspecifically activated alveolar macrophages ingest the bacilli. Invasion of macrophages by mycobacteria may result in part from association of C2a with the bacterial cell wall followed by C3b opsonization of the bacteria and recognition by the macrophages. The balance between the bactericidal activity of the macrophage and the virulence of the bacillus (the latter being partially linked to the bacterium's lipid-rich cell wall and to its glycolipid capsule, both of which confer resistance to complement and free radicals of the phagocyte) determines the events following phagocytosis. The number of invading bacilli is also important.
Several genes thought to confer virulence to M. tuberculosis have been identified. katG encodes for catalase, an enzyme protective against oxidative stress; rpoV is the main sigma factor initiating transcription of several genes. Defects in these two genes result in loss of virulence. The erp gene, encoding a protein required for multiplication, also contributes to virulence. The effects of a highly virulent strain are exemplified by an outbreak of tuberculosis in two rural counties in Tennessee and Kentucky in 1994 through 1996. In this outbreak, both epidemiologic evidence of enhanced transmission with high rates of disease and accelerated growth of the strain in mice were documented.
Several observations suggest that genetic factors play a key role in innate nonimmune resistance to infection with M. tuberculosis. The existence of this resistance is suggested by the differing degrees of susceptibility to tuberculosis in different populations. In mice, a gene called Nramp1 (natural resistance-associated macrophage protein 1) has a regulatory role in resistance/susceptibility to mycobacteria. The human homologue NRAMP1, cloned to chromosome 2q, may have a role in determining susceptibility to tuberculosis. In a study among West Africans, subjects heterozygous for two polymorphisms of NRAMP1 (INT4 and 3′UTR) had an apparently increased risk of tuberculosis, a finding suggesting that the susceptibility allele behaves as dominant.
In the initial stage of host-bacterium interaction, either the host's macrophages contain bacillary multiplication by producing proteolytic enzymes and cytokines or the bacilli begin to multiply. If the bacilli multiply, their growth quickly kills the macrophages, which lyse. Nonactivated monocytes attracted from the bloodstream to the site by various chemotactic factors ingest the bacilli released from the lysed macrophages. These initial stages of infection are usually asymptomatic.
About 2 to 4 weeks after infection, two additional host responses to M. tuberculosis develop: a tissue-damaging response and a macrophage-activating response. The tissue-damaging response is the result of a delayed-type hypersensitivity (DTH) reaction to various bacillary antigens; it destroys nonactivated macrophages that contain multiplying bacilli. The macrophage-activating response is a cell-mediated phenomenon resulting in the activation of macrophages that are capable of killing and digesting tubercle bacilli. Although both of these responses can inhibit mycobacterial growth (Fig. 169-1), it is the balance between the two that determines the form of tuberculosis that will develop subsequently.

Figure 169-1:
Chest radiograph of a 39-year-old man with acute upper lobe tuberculosis demonstrating typical cavitary infiltrates. Apical localization of pulmonary tuberculosis is characteristic of adult infection. This localization has been attributed to the hyperoxic environment of the apices, but another theory proposes that lymph production is deficient at the apices, favoring retention of antigens at this location. (Courtesy of N Ettinger, MD. )(RA Goodwin, RM des Prez: Apical localization of pulmonary tuberculosis, chronic pulmonary histoplasmosis, and progressive massive fibrosis of the lung. Chest 83:801-805, 1983.)With the development of specific immunity and the accumulation of large numbers of activated macrophages at the site of the primary lesion, granulomatous lesions (tubercles) are formed. These lesions consist of lymphocytes and activated macrophages, such as epithelioid cells and giant cells. Initially, the newly developed tissue-damaging response is the only event capable of limiting mycobacterial growth within macrophages. This response, mediated by various bacterial products, not only destroys macrophages but also produces early solid necrosis in the center of the tubercle. Although M. tuberculosis can survive, its growth is inhibited within this necrotic environment by low oxygen tension and low pH. At this point, some lesions may heal by fibrosis and calcification, while others undergo further evolution.
Cell-mediated immunity is critical at this early stage. In the majority of infected individuals, local macrophages are activated when bacillary antigens processed by macrophages stimulate T lymphocytes to release a variety of lymphokines. These activated cells aggregate around the lesion's center and effectively neutralize tubercle bacilli without causing further tissue destruction. In the central part of the lesion, the necrotic material resembles soft cheese (caseous necrosis). Even when healing takes place, viable bacilli may remain dormant within macrophages or in the necrotic material for years or even throughout the patient's lifetime. These "healed" lesions in the lung parenchyma and hilar lymph nodes may later undergo calcification (Ranke complex).
In a minority of cases, the macrophage-activating response is weak, and mycobacterial growth can be inhibited only by intensified DTH reactions, which lead to tissue destruction. The lesion tends to enlarge further, and the surrounding tissue is progressively damaged. At the center of the lesion, the caseous material liquefies. Bronchial walls as well as blood vessels are invaded and destroyed, and cavities are formed. The liquefied caseous material, containing large numbers of bacilli, is drained through bronchi. Within the cavity, tubercle bacilli multiply well and spread into the airways and the environment through expectorated sputum.
In the early stages of infection, bacilli are usually transported by macrophages to regional lymph nodes, from which they disseminate widely to many organs and tissues. The resulting lesions may undergo the same evolution as those in the lungs, although most tend to heal. In young children with poor natural immunity, hematogenous dissemination may result in fatal miliary tuberculosis or tuberculous meningitis.
Cell-mediated immunity confers partial protection against M. tuberculosis, while humoral immunity has no defined role in protection. Two types of cells are essential: macrophages, which directly phagocytize tubercle bacilli, and T cells (mainly CD4+ lymphocytes), which induce protection through the production of lymphokines.
After infection with M. tuberculosis, alveolar macrophages secrete a number of cytokines: interleukin (IL) 1 contributes to fever; IL-6 contributes to hyperglobulinemia; and tumor necrosis factor ![]() (TNF-
(TNF-![]() ) contributes to the killing of mycobacteria, the formation of granulomas, and a number of systemic effects, such as fever and weight loss. Macrophages are also critical in processing and presenting antigens to T lymphocytes; the result is a proliferation of CD4+ lymphocytes, which are crucial to the host's defense against M. tuberculosis. Qualitative and quantitative defects of CD4+ T cells explain the inability of HIV-infected individuals to contain mycobacterial proliferation. Reactive CD4+ lymphocytes produce cytokines of the TH1 pattern and participate in MHC class II-restricted killing of cells infected with M. tuberculosis. TH1 CD4+ cells produce interferon
) contributes to the killing of mycobacteria, the formation of granulomas, and a number of systemic effects, such as fever and weight loss. Macrophages are also critical in processing and presenting antigens to T lymphocytes; the result is a proliferation of CD4+ lymphocytes, which are crucial to the host's defense against M. tuberculosis. Qualitative and quantitative defects of CD4+ T cells explain the inability of HIV-infected individuals to contain mycobacterial proliferation. Reactive CD4+ lymphocytes produce cytokines of the TH1 pattern and participate in MHC class II-restricted killing of cells infected with M. tuberculosis. TH1 CD4+ cells produce interferon ![]() (IFN-
(IFN-![]() ) and IL-2 and promote cell-mediated immunity. TH2 cells produce IL-4, IL-5, and IL-10 and promote humoral immunity. The interplay of these various cytokines and their cross-regulation determine the host's response. The role of cytokines in promoting intracellular killing of mycobacteria has not been entirely elucidated. IFN-
) and IL-2 and promote cell-mediated immunity. TH2 cells produce IL-4, IL-5, and IL-10 and promote humoral immunity. The interplay of these various cytokines and their cross-regulation determine the host's response. The role of cytokines in promoting intracellular killing of mycobacteria has not been entirely elucidated. IFN-![]() may induce release of nitric oxide, and TNF-
may induce release of nitric oxide, and TNF-![]() seems also to be important. Finally, the role of other cells, such as natural killer (NK) cells, "double-negative" CD4-CD8- cells, and
seems also to be important. Finally, the role of other cells, such as natural killer (NK) cells, "double-negative" CD4-CD8- cells, and ![]() /
/![]() T cells, in protective immunity remains unclear.
T cells, in protective immunity remains unclear.
M. tuberculosis possesses various protein antigens. Some are present in the cytoplasm and cell wall; others are secreted. That the latter are more important in eliciting a T lymphocyte response is suggested by experiments documenting the appearance of protective immunity in animals after immunization with live, protein-secreting mycobacteria. Among the antigens with a potential protective role are the 30-kDa (or 85B) and the ESAT-6 antigens. Protective immunity is probably the result of reactivity to a large number of different mycobacterial antigens.
Coincident with the appearance of immunity, DTH to M. tuberculosis develops. This reactivity is the basis of the PPD skin test, currently the only test that reliably detects M. tuberculosis infection in persons without symptoms. The cellular mechanisms responsible for PPD reactivity are related mainly to previously sensitized CD4+ lymphocytes, which are attracted to the skin-test site. There, they proliferate and produce cytokines.
In 1891, Robert Koch discovered components of M. tuberculosis in a concentrated liquid culture medium. Subsequently named "old tuberculin" (OT), this material was initially believed to be useful in the treatment of tuberculosis (although this idea was later disproved). It soon became clear that OT was capable of eliciting a skin reaction when injected subcutaneously into patients with tuberculosis. In 1932, Seibert and Munday purified this product by ammonium sulfate precipitation. The result was an active protein fraction known as tuberculin PPD. However, the complexity and diversity of the constituents of PPD rendered its standardization difficult. PPD-S, developed by Seibert and Glenn in 1941, was chosen as the international standard. Later, the WHO and UNICEF sponsored large-scale production of a master batch of PPD, termed RT23, and made it available for general use. The greatest limitation of PPD is its lack of mycobacterial species specificity, a property that is due to the large number of proteins in this product that are highly conserved in the various species of mycobacteria.
While DTH is associated with protective immunity (PPD-positive persons being less susceptible to a new M. tuberculosis infection than PPD-negative persons), it by no means guarantees protection against reactivation. In fact, severe cases of active tuberculosis are often accompanied by strongly positive skin-test reactions.
Clinical Manifestations
Tuberculosis is usually classified as pulmonary or extrapulmonary. Before the recognition of HIV infection, more than 80% of all cases of tuberculosis were limited to the lungs. However, up to two-thirds of HIV-infected patients with tuberculosis may have both pulmonary and extrapulmonary disease or extrapulmonary disease alone.
Pulmonary Tuberculosis
Pulmonary tuberculosis can be categorized as primary or postprimary (secondary).
Primary Disease
Primary pulmonary tuberculosis results from an initial infection with tubercle bacilli. In areas of high tuberculosis prevalence, this form of disease is often seen in children and is frequently localized to the middle and lower lung zones. The lesion forming after infection is usually peripheral and accompanied by hilar or paratracheal lymphadenopathy, which may not be detectable on chest radiography. In the majority of cases, the lesion heals spontaneously and may later be evident as a small calcified nodule (Ghon lesion).
In children and in persons with impaired immunity, such as those with malnutrition or HIV infection, primary pulmonary tuberculosis may progress rapidly to clinical illness. The initial lesion increases in size and can evolve in different ways. Pleural effusion, a frequent finding, results from the penetration of bacilli into the pleural space from an adjacent subpleural focus. In severe cases, the primary site rapidly enlarges, its central portion undergoes necrosis, and acute cavitation develops (progressive primary tuberculosis). Tuberculosis in young children is almost invariably accompanied by hilar or mediastinal lymphadenopathy due to the spread of bacilli from the lung parenchyma through lymphatic vessels. Enlarged lymph nodes may compress bronchi, causing obstruction and subsequent segmental or lobar collapse. Partial obstruction may cause obstructive emphysema, and bronchiectasis may also develop. Hematogenous dissemination, which is common and is often asymptomatic, may result in the most severe manifestations of primary M. tuberculosis infection. Bacilli reach the bloodstream from the pulmonary lesion or the lymph nodes and disseminate into various organs, where they may produce granulomatous lesions. Although healing frequently takes place, immunocompromised persons (e.g., patients with HIV infection and those recovering from measles) may develop miliary tuberculosis and/or tuberculous meningitis.
Postprimary Disease
Also called adult-type, reactivation, or secondary tuberculosis, postprimary disease results from endogenous reactivation of latent infection and is usually localized to the apical and posterior segments of the upper lobes, where the high oxygen concentration favors mycobacterial growth. In addition, the superior segments of the lower lobes are frequently involved. The extent of lung parenchymal involvement varies greatly, from small infiltrates to extensive cavitary disease. With cavity formation, liquefied necrotic contents are ultimately discharged into the airways, resulting in satellite lesions within the lungs that may in turn undergo cavitation. Massive involvement of pulmonary segments or lobes, with coalescence of lesions, produces tuberculous pneumonia. While up to one-third of untreated patients reportedly succumb to severe pulmonary tuberculosis within a few weeks or months after onset, others undergo a process of spontaneous remission or proceed along a chronic, progressively debilitating course ("consumption"). Under these circumstances, some pulmonary lesions become fibrotic and may later calcify, but cavities persist in other parts of the lungs. Individuals with such chronic disease continue to discharge tubercle bacilli into the environment. Most patients respond to treatment, with defervescence, decreasing cough, weight gain, and a general improvement in well-being within several weeks.
Early in the course of disease, symptoms and signs are often nonspecific and insidious, consisting mainly of fever and night sweats, weight loss, anorexia, general malaise, and weakness. However, in the majority of cases, cough eventually develops-perhaps initially nonproductive and subsequently accompanied by the production of purulent sputum. Blood streaking of the sputum is frequently documented. Massive hemoptysis may ensue as a consequence of the erosion of a fully patent vessel located in the wall of a cavity. Hemoptysis, however, may also result from rupture of a dilated vessel in a cavity (Rasmussen's aneurysm) or from aspergilloma formation in an old cavity. Pleuritic chest pain sometimes develops in patients with subpleural parenchymal lesions but can also result from muscle strain due to persistent coughing. Extensive disease may produce dyspnea and (occasionally) adult respiratory distress syndrome (ARDS).
Physical findings are of limited use in pulmonary tuberculosis. Many patients have no abnormalities detectable by chest examination, while others have detectable rales in the involved areas during inspiration, especially after coughing. Occasionally, rhonchi due to partial bronchial obstruction and classic amphoric breath sounds in areas with large cavities may be heard. Systemic features include fever (often low-grade and intermittent) and wasting. In some cases, pallor and finger clubbing develop. The most common hematologic findings are mild anemia and leukocytosis. Hyponatremia due to the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) has also been reported.
Extrapulmonary Tuberculosis
In order of frequency, the extrapulmonary sites most commonly involved in tuberculosis are the lymph nodes, pleura, genitourinary tract, bones and joints, meninges, and peritoneum. However, virtually all organ systems may be affected. As a result of hematogenous dissemination in HIV-infected individuals, extrapulmonary tuberculosis is seen more commonly today than in the past.
Lymph-Node Tuberculosis (Tuberculous Lymphadenitis)
The commonest presentation of extrapulmonary tuberculosis (being documented in more than 25% of cases), lymph-node disease is particularly frequent among HIV-infected patients. In the United States, children and women (particularly non-Caucasians) also seem to be especially susceptible. Lymph-node tuberculosis presents as painless swelling of the lymph nodes, most commonly at cervical and supraclavicular sites. Lymph nodes are usually discrete in early disease but may be inflamed and have a fistulous tract draining caseous material (Fig. 169-2). Systemic symptoms are usually limited to HIV-infected patients, and concomitant lung disease may or may not be present. The diagnosis is established by fine-needle aspiration or surgical biopsy. AFB are seen in up to 50% of cases, cultures are positive in 70 to 80%, and histologic examination shows granulomatous lesions. Among HIV-infected patients, granulomas are usually not seen. Differential diagnosis includes a variety of infectious conditions as well as neoplastic diseases such as lymphomas or metastatic carcinomas (Chap. 63).

Figure 169-2:
Scrofuloderma Large abscess near the clavicular head, which on pressure extrudes pus and caseous material. A tuberculous lymphadenitis undergoes necrosis with abscess formation, subsequently draining to the skin sll1face.Pleural Tuberculosis
Involvement of the pleura is common in primary tuberculosis and results from penetration by a few tubercle bacilli into the pleural space. Depending on the extent of reactivity, the effusion may be small, remain unnoticed, and resolve spontaneously or may be sufficiently large to cause symptoms such as fever, pleuritic chest pain, and dyspnea. Physical findings are those of pleural effusion: dullness to percussion and absence of breath sounds. A chest radiograph reveals the effusion and, in no more than one-third of cases, also shows a parenchymal lesion. Thoracentesis is required to ascertain the nature of the effusion. The fluid is straw colored and at times hemorrhagic; it is an exudate with a protein concentration >50% of that in serum, a normal to low glucose concentration, a pH that is generally <7.2, and detectable white blood cells (usually 500 to 2500/mL). Neutrophils may predominate in the early stage, while mononuclear cells are the typical finding later. Mesothelial cells are generally rare or absent. AFB are very rarely seen on direct smear, but cultures may be positive for M. tuberculosis in up to one-third of cases. Needle biopsy of the pleura is often required for diagnosis and reveals granulomas and/or yields a positive culture in up to 70% of cases. This form of pleural tuberculosis responds well to chemotherapy and may resolve spontaneously.
Tuberculous empyema is a less common complication of pulmonary tuberculosis. It is usually the result of the rupture of a cavity, with delivery of a large number of organisms into the pleural space, or of a bronchopleural fistula from a pulmonary lesion. A chest radiograph may show pyopneumothorax with an air-fluid level. The effusion is purulent and thick and contains large numbers of lymphocytes. An acid-fast smear of pleural fluid is often found to be positive when examined by microscopy, as is culture of the pleural fluid. Surgical drainage is usually required as an adjunct to chemotherapy. Tuberculous empyema may result in severe pleural fibrosis and restrictive lung disease.
Tuberculosis of the Upper Airways
Nearly always a complication of advanced cavitary pulmonary tuberculosis (Fig. 169-3), tuberculosis of the upper airways may involve the larynx, pharynx, and epiglottis. Symptoms include hoarseness and dysphagia in addition to chronic productive cough. Findings depend on the site of involvement, and ulcerations may be seen on laryngoscopy. Acid-fast smear of the sputum is often positive, but biopsy may be necessary in some cases to establish the diagnosis. Cancer may have similar features but is usually painless.

Figure 169-3:
Orificial tuberculosis A large, very painful ulcer on the lips of this patient with advanced cavitary pulmonary tuberculosis.Genitourinary Tuberculosis
Genitourinary tuberculosis accounts for about 15% of all extrapulmonary cases, may involve any portion of the genitourinary tract, and is usually due to hematogenous seeding following primary infection. Local symptoms predominate. Urinary frequency, dysuria, hematuria, and flank pain are common presentations. However, patients may be asymptomatic and the disease discovered only after severe destructive lesions of the kidneys have developed. Urinalysis gives abnormal results in 90% of cases, revealing pyuria and hematuria. The documentation of culture-negative pyuria in acidic urine raises the suspicion of tuberculosis. An intravenous pyelogram helps in diagnosis. Calcifications and ureteral strictures are suggestive findings. Culture of three morning urine specimens yields a definitive diagnosis in nearly 90% of cases. Severe ureteral strictures may lead to hydronephrosis and renal damage.
Genital tuberculosis is diagnosed more commonly in females than in males. In females, it affects the fallopian tubes and the endometrium and may cause infertility, pelvic pain, and menstrual abnormalities. Diagnosis requires biopsy or culture of specimens obtained by dilatation and curettage. In males, tuberculosis preferentially affects the epididymis, producing a slightly tender mass that may drain externally through a fistulous tract; orchitis and prostatitis may also develop. In almost half of cases of genitourinary tuberculosis, urinary tract disease is also present. Genitourinary tuberculosis responds well to chemotherapy.
Skeletal Tuberculosis
In the United States, tuberculosis of the bones and joints is responsible for about 10% of extrapulmonary cases. In bone and joint disease, pathogenesis is related to reactivation of hematogenous foci or to spread from adjacent paravertebral lymph nodes. Weight-bearing joints (spine, hips, and knees-in that order) are affected most commonly. Spinal tuberculosis (Pott's disease or tuberculous spondylitis) often involves two or more adjacent vertebral bodies. While the upper thoracic spine is the most common site of spinal tuberculosis in children, the lower thoracic and upper lumbar vertebrae are usually affected in adults. From the anterior superior or inferior angle of the vertebral body, the lesion reaches the adjacent body, also destroying the intervertebral disk. With advanced disease, collapse of vertebral bodies results in kyphosis (gibbus). A paravertebral "cold" abscess may also form. In the upper spine, this abscess may track to the chest wall as a mass; in the lower spine, it may reach the inguinal ligaments or present as a psoas abscess. Computed tomography (CT) or magnetic resonance imaging (MRI) reveals the characteristic lesion and suggests its etiology, although the differential diagnosis includes other infections and tumors. Aspiration of the abscess or bone biopsy confirms the tuberculous etiology, as cultures are usually positive and histologic findings highly typical. A catastrophic complication of Pott's disease is paraplegia, which is usually due to an abscess or a lesion compressing the spinal cord. Paraparesis due to a large abscess is a medical emergency and requires abscess drainage. Tuberculosis of the hip joints causes pain and limping; tuberculosis of the knee produces pain and swelling and sometimes follows trauma. If the disease goes unrecognized, the joints may be destroyed. Skeletal tuberculosis responds to chemotherapy, but severe cases may require surgery.
Tuberculous Meningitis and Tuberculoma
Tuberculosis of the central nervous system accounts for about 5% of extrapulmonary cases. It is seen most often in young children but also develops in adults, especially those who are infected with HIV. Tuberculous meningitis results from the hematogenous spread of primary or postprimary pulmonary disease or from the rupture of a subependymal tubercle into the subarachnoid space. In more than half of cases, evidence of old pulmonary lesions or a miliary pattern is found on chest radiography. The disease may present subtly as headache and mental changes or acutely as confusion, lethargy, altered sensorium, and neck rigidity. Typically, the disease evolves over 1 or 2 weeks, a course longer than that of bacterial meningitis. Paresis of cranial nerves (ocular nerves in particular) is a frequent finding, and the involvement of cerebral arteries may produce focal ischemia. Hydrocephalus is common. Lumbar puncture is the cornerstone of diagnosis. In general, examination of the cerebrospinal fluid (CSF) reveals a high leukocyte count (usually with a predominance of lymphocytes but often with a predominance of neutrophils in the early stage), a protein content of 1 to 8 g/L (100 to 800 mg/dL), and a low glucose concentration; however, any of these three parameters can be within the normal range. AFB are seen on direct smear of CSF sediment in only 20% of cases, but repeated lumbar punctures increase the yield. Culture of CSF is diagnostic in up to 80% of cases. Imaging studies (CT and MRI) may show hydrocephalus and abnormal enhancement of basal cisterns or ependyma. If unrecognized, tuberculous meningitis is uniformly fatal. This disease responds to chemotherapy; however, neurologic sequelae are documented in 25% of treated cases, in most of which the diagnosis has been delayed. Clinical trials have demonstrated that patients treated with adjunctive glucocorticoids experience a significantly faster resolution of CSF abnormalities and elevated CSF pressure. Adjunctive glucocorticoids enhance survival and reduce the frequency of neurologic sequelae.
Tuberculoma, an uncommon manifestation of tuberculosis, presents as one or more space-occupying lesions and usually causes seizures and focal signs. CT or MRI reveals contrast-enhanced ring lesions, but biopsy is necessary to establish the diagnosis.
Gastrointestinal Tuberculosis
Any portion of the gastrointestinal tract may be affected by tuberculosis. Various pathogenetic mechanisms are involved: swallowing of sputum with direct seeding, hematogenous spread, or (although rarely today) ingestion of milk from cows affected by bovine tuberculosis. The terminal ileum and the cecum are the sites most commonly involved. Abdominal pain (at times similar to that associated with appendicitis), diarrhea, obstruction, hematochezia, and a palpable mass in the abdomen are common findings at presentation. Fever, weight loss, and night sweats are also frequent. With intestinal-wall involvement, ulcerations and fistulae may simulate Crohn's disease. Anal fistulae should prompt an evaluation for rectal tuberculosis. As surgery is required in most cases, the diagnosis can be established by histologic examination and culture of specimens obtained intraoperatively.
Tuberculous peritonitis follows either the direct spread of tubercle bacilli from ruptured lymph nodes and intraabdominal organs or hematogenous seeding. Nonspecific abdominal pain, fever, and ascites should raise the suspicion of tuberculous peritonitis. The coexistence of cirrhosis (Chap. 298) in patients with tuberculous peritonitis complicates the diagnosis. In tuberculous peritonitis, paracentesis reveals an exudative fluid with a high protein content and leukocytosis that is usually lymphocytic (although neutrophils occasionally predominate). The yield of direct smear and culture is relatively low; culture of a large volume of ascitic fluid can increase the yield, but peritoneal biopsy is often needed to establish the diagnosis.
Pericardial Tuberculosis (Tuberculous Pericarditis)
Due to direct progression of a primary focus within the pericardium, to reactivation of a latent focus, or to rupture of an adjacent lymph node, pericardial tuberculosis has often been a disease of the elderly in countries with low tuberculosis prevalence but develops frequently in HIV-infected patients. Case-fatality rates are as high as 40% in some series. The onset may be subacute, although an acute presentation, with fever, dull retrosternal pain, and a friction rub, is possible. An effusion eventually develops in many cases; cardiovascular symptoms and signs of cardiac tamponade may ultimately appear (Chap. 239). In the presence of effusion detected on chest radiography, tuberculosis must be suspected if the patient belongs to a high-risk population (HIV-infected, originating in a high-prevalence country), if there is evidence of previous tuberculosis or disease in other organs, or if echocardiography shows thick strands crossing the pericardial space. Diagnosis can be facilitated by pericardiocentesis under echocardiographic guidance. The pericardial fluid must be submitted for biochemical, cytologic, and microbiologic study. The effusion is exudative in nature, with a high count of leukocytes (predominantly mononuclear cells). Hemorrhagic effusion is frequent. Culture of the fluid reveals M. tuberculosis in about 30% of cases, while biopsy has a higher yield. Without treatment, pericardial tuberculosis is usually fatal. Even with treatment, complications may develop, including chronic constrictive pericarditis with thickening of the pericardium, fibrosis, and sometimes calcification, which may be visible on a chest radiograph. A course of glucocorticoid treatment is useful in the management of acute disease, reducing effusion, facilitating hemodynamic recovery, and thus decreasing mortality. Progression to chronic constrictive pericarditis, however, seems unaffected by such therapy.
Miliary or Disseminated Tuberculosis
Miliary tuberculosis is due to hematogenous spread of tubercle bacilli. Although in children it is often the consequence of a recent primary infection, in adults it may be due to either recent infection or reactivation of old disseminated foci. Lesions are usually yellowish granulomas 1 to 2 mm in diameter that resemble millet seeds (thus the term miliary, coined by nineteenth-century pathologists).
Clinical manifestations are nonspecific and protean, depending on the predominant site of involvement. Fever, night sweats, anorexia, weakness, and weight loss are presenting symptoms in the majority of cases. At times, patients have a cough and other respiratory symptoms due to pulmonary involvement as well as abdominal symptoms. Physical findings include hepatomegaly, splenomegaly, and lymphadenopathy. Eye examination may reveal choroidal tubercles, which are pathognomonic of miliary tuberculosis, in up to 30% of cases. Meningismus occurs in fewer than 10% of cases.
A high index of suspicion is required for the diagnosis of miliary tuberculosis. Frequently, chest radiography (Fig. 169-5) reveals a miliary reticulonodular pattern (more easily seen on underpenetrated film), although no radiographic abnormality may be evident early in the course and among HIV-infected patients. Other radiologic findings include large infiltrates, interstitial infiltrates (especially in HIV-infected patients), and pleural effusion. A sputum smear is negative in 80% of cases. Various hematologic abnormalities may be seen, including anemia with leukopenia, neutrophilic leukocytosis and leukemoid reactions, and polycythemia. Disseminated intravascular coagulation has been reported. Elevation of alkaline phosphatase levels and other abnormal values in liver function tests are detected in patients with severe hepatic involvement. The PPD test may be negative in up to half of cases, but reactivity may be restored during chemotherapy. Bronchoalveolar lavage and transbronchial biopsy are more likely to permit bacteriologic confirmation, and granulomas are evident in liver or bone-marrow biopsy specimens from many patients. If it goes unrecognized, miliary tuberculosis is lethal; with proper treatment, however, it is amenable to cure.

Figure 169-5:
Chest radiograph of miliary tuberculosis showing widespread, small, discrete densities. Hematogenous dissemination of infection occurs before development of hypersensitivity. In the vast majority of people, dissemination stops when hypersensitivity develops, but in some individuals, it progresses to the clinical syndrome of acute miliary disease. (Courtesy of N Ettinger, MD.)A rare presentation seen in the elderly is cryptic miliary tuberculosis, which has a chronic course characterized by mild intermittent fever, anemia, and-ultimately-meningeal involvement preceding death. An acute septicemic form, nonreactive miliary tuberculosis, occurs very rarely and is due to massive hematogenous dissemination of tubercle bacilli. Pancytopenia is common in this form of disease, which is rapidly fatal. At postmortem examination, multiple necrotic but nongranulomatous ("nonreactive") lesions are detected.
Less Common Extrapulmonary Forms
Tuberculosis may cause chorioretinitis, uveitis, panophthalmitis, and painful hypersensitivity-related phlyctenular conjunctivitis. Tuberculous otitis is rare and presents as hearing loss, otorrhea, and tympanic membrane perforation. In the nasopharynx, tuberculosis may simulate Wegener's granulomatosis. Cutaneous manifestations of tuberculosis include primary infection due to direct inoculation, abscesses and chronic ulcers, scrofuloderma (Fig. 169-2), lupus vulgaris (Fig. 169-4), miliary lesions, and erythema nodosum. Adrenal tuberculosis is a manifestation of advanced disease presenting as signs of adrenal insufficiency. Finally, congenital tuberculosis results from transplacental spread of tubercle bacilli to the fetus or from ingestion of contaminated amniotic fluid. This rare disease affects the liver, spleen, lymph nodes, and various other organs.
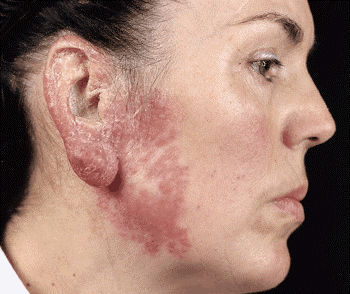
Figure 169-4:
Lupus vulgaris Reddish-brown plaque, which on diascopy exhibits the diagnostic yellow-brown apple-jelly color. Note nodular infiltration of the earlobe, scaling of the helix, and atrophic scarring in the center of the plaque.HIV-Associated Tuberculosis
Tuberculosis is an important opportunistic disease among HIV-infected persons worldwide. In developing countries of Africa, Southeast Asia, and Latin America, an estimated 10 million persons were coinfected as of 1997. In the United States, coinfection with HIV and M. tuberculosis is common in certain segments of the population, including drug users and some minorities. A person with skin test-documented M. tuberculosis infection who acquires HIV infection has a 3 to 15% annual risk of developing active tuberculosis.
The association between tuberculosis and HIV infection is supported by other epidemiologic observations. First, the rate of HIV seropositivity among patients with tuberculosis is several times higher than that among the general population: in African countries it reaches 60 to 70%. Second, marked increases in numbers of tuberculosis cases have been reported at locations hard hit by the HIV epidemic, such as large parts of Africa (including Kenya, Tanzania, and Malawi), northern Thailand, and New York City. Globally, the proportion of tuberculosis cases associated with HIV infection reached 8% in 1997.
HIV directly attacks the critical immune mechanisms involved in protection against tuberculosis. Tuberculosis can appear at any stage of HIV infection, but its presentation varies with the stage. When cell-mediated immunity is only partially compromised, pulmonary tuberculosis presents as a typical pattern of upper lobe infiltrates and cavitation, without significant lymphadenopathy or pleural effusion. In late stages of HIV infection, a primary tuberculosis-like pattern, with diffuse interstitial or miliary infiltrates, little or no cavitation, and intrathoracic lymphadenopathy, is more common. Overall, sputum smears may be positive less frequently among tuberculosis patients with HIV infection than among those without; thus the diagnosis of tuberculosis may be unusually difficult, especially in view of the variety of HIV-related pulmonary conditions mimicking tuberculosis.
As has been mentioned, extrapulmonary tuberculosis is common among HIV-infected patients. In various series studied in the United States and many developing countries, extrapulmonary tuberculosis-alone or in association with pulmonary disease-has been documented in 40 to 60% of all cases in HIV co-infected individuals. The most common forms are lymphatic, disseminated, pleural, and pericardial. Mycobacteremia and meningitis are also frequent, particularly in advanced HIV disease.
The diagnosis of tuberculosis in HIV-infected patients may be difficult not only because of the increased frequency of sputum-smear negativity (up to 40% in culture-proven pulmonary cases) but also because of atypical radiographic findings, a lack of classic granuloma formation in the late stages, and negative results in PPD skin tests. Delays in treatment may prove fatal. The response to short-course chemotherapy is similar to that in HIV-seronegative patients. However, adverse effects may be more pronounced, including severe or even fatal skin reactions to amithiozone (thiacetazone).
Exacerbations in symptoms, signs, and laboratory or radiographic manifestations of tuberculosis-termed paradoxical reactions-have been associated with the administration of highly active antiretroviral treatment (HAART) regimens. The presumed pathogenesis of paradoxical reactions is an immune response to antigens released as bacilli are killed by effective chemotherapy. In patients in whom antiretroviral therapy has recently been started, paradoxical reactions may be due to improving immune function. The first priority in the management of a possible paradoxical reaction is to ensure that the clinical syndrome does not represent a failure of tuberculosis treatment or the development of another infection. Mild paradoxical reactions can be managed with symptom-based treatment. More severe manifestations may necessitate discontinuation of antiretroviral therapy. Immunomodulators, such as glucocorticoids, have been used to treat severe paradoxical reactions, although this practice has not been formally evaluated in clinical trials.
Recommendations for the prevention and treatment of tuberculosis in HIV-infected individuals have been published by the CDC.
Diagnosis
The key to the diagnosis of tuberculosis is a high index of suspicion. Diagnosis is not difficult with a high-risk patient-e.g., a homeless alcoholic who presents with typical symptoms and a classic chest radiograph showing upper lobe infiltrates with cavities. On the other hand, the diagnosis can easily be missed in an elderly nursing-home resident or a teenager with a focal infiltrate.
Often, the diagnosis is first entertained when the chest radiograph of a patient being evaluated for respiratory symptoms is abnormal. If the patient has no complicating medical conditions that favor immunosuppression, the chest radiograph may show the typical picture of upper lobe infiltrates with cavitation. The longer the delay between the onset of symptoms and the diagnosis, the more likely is the finding of cavitary disease. In contrast, immunosuppressed patients, including those with HIV infection, may have "atypical" findings on chest radiography-e.g., lower zone infiltrates without cavity formation.
AFB Microscopy
A presumptive diagnosis is commonly based on the finding of AFB on microscopic examination of a diagnostic specimen such as a smear of expectorated sputum or of tissue (for example, a lymph node biopsy). Most modern laboratories processing large numbers of diagnostic specimens use auramine-rhodamine staining and fluorescence microscopy. The more traditional method-light microscopy of specimens stained with Kinyoun or Ziehl-Neelsen basic fuchsin dyes-is satisfactory, although more time-consuming. For patients with suspected pulmonary tuberculosis, three sputum specimens, preferably collected early in the morning, should be submitted to the laboratory for AFB smear and mycobacteriology culture. If tissue is obtained, it is critical that the portion of the specimen intended for culture not be put in formaldehyde. The use of AFB microscopy on urine or gastric lavage fluid is limited by the presence of mycobacterial commensals, which can cause false-positive results.
Mycobacterial Culture
Definitive diagnosis depends on the isolation and identification of M. tuberculosis from a diagnostic specimen-in most cases, a sputum specimen obtained from a patient with a productive cough. Specimens may be inoculated onto egg- or agar-based medium (e.g., Löwenstein-Jensen or Middlebrook 7H10) and incubated at 37°C under 5% CO2. Because most species of mycobacteria, including M. tuberculosis, grow slowly, 4 to 8 weeks may be required before growth is detected. Although M. tuberculosis may be presumptively identified on the basis of growth time and colony pigmentation and morphology, a variety of biochemical tests have traditionally been used to speciate mycobacterial isolates. In today's laboratories, the use of liquid media with radiometric growth detection (e.g., BACTEC-460) and the identification of isolates by nucleic acid probes or high-pressure liquid chromatography of mycolic acids have replaced the traditional methods of isolation on solid media and identification by biochemical tests. These new methods have decreased the time required for isolation and speciation to 2 to 3 weeks. Other systems for culture on liquid media with nonradiometric detection have become available.
Nucleic Acid Amplification
Several test systems based on amplification of mycobacterial nucleic acid are available. These systems permit the diagnosis of tuberculosis in as short a period as several hours. However, their applicability is limited by low sensitivity (lower than culture) and high cost. At present, these tests are approved by the U.S. Food and Drug Administration only for species identification on AFB-positive sputa. With further improvements in performance, these tests may also be useful for the diagnosis of patients with AFB-negative pulmonary and extrapulmonary tuberculosis.
Radiographic Procedures
As noted above, the initial suspicion of pulmonary tuberculosis is often based on abnormal chest radiographic findings in a patient with respiratory symptoms. Although the "classic" picture is that of upper lobe disease with infiltrates and cavities, virtually any radiographic pattern-from a normal film or a solitary pulmonary nodule to diffuse alveolar infiltrates in a patient with ARDS-may be seen. In the era of AIDS, no radiographic pattern can be considered pathognomonic.
PPD Skin Testing
Skin testing with PPD is most widely used in screening for M. tuberculosis infection (see below). The test is of limited value in the diagnosis of active tuberculosis because of its low sensitivity and specificity. False-negative reactions are common in immunosuppressed patients and in those with overwhelming tuberculosis. Positive reactions are obtained when patients have been infected with M. tuberculosis but do not have active disease and when persons have been sensitized by nontuberculous mycobacteria (Chap. 171) or bacille Calmette-Guérin (BCG) vaccination. Although BCG vaccine is not commonly used in the United States, many immigrants will have received it. In the absence of a history of BCG vaccination, a positive skin test may provide additional support for the diagnosis of tuberculosis in culture-negative cases.
Drug Susceptibility Testing
In general, the initial isolate of M. tuberculosis should be tested for susceptibility to the primary drugs used for treatment: isoniazid, rifampin, ethambutol, pyrazinamide, and streptomycin. In addition, drug susceptibility tests are mandatory when patients fail to respond to initial therapy or experience a relapse after the completion of treatment (see below). Susceptibility testing may be conducted directly (with the clinical specimen) or indirectly (with mycobacterial cultures) on solid or liquid medium. Results are obtained most rapidly by direct susceptibility testing on liquid medium, with an average reporting time of 3 weeks. With indirect testing on solid media, results may not be available for 8 weeks or longer. Molecular methods for the rapid identification of drug resistance are becoming available. One of the most promising uses polymerase chain reaction (PCR) for the rpoB gene to detect resistance to rifampin.
Additional Diagnostic Procedures
Other diagnostic tests may be used when pulmonary tuberculosis is suspected. Sputum induction by ultrasonic nebulization of hypertonic saline may be useful for patients unable to produce a sputum specimen spontaneously. Frequently, patients with radiographic abnormalities that are consistent with other diagnoses (e.g., bronchogenic carcinoma) undergo fiberoptic bronchoscopy with bronchial brushings or transbronchial biopsy of the lesion. Bronchoalveolar lavage of a lung segment containing an abnormality may also be performed. In all cases, it is essential that specimens be submitted for AFB smear and mycobacterial culture. For the diagnosis of primary pulmonary tuberculosis in children, who often do not expectorate sputum, specimens from early-morning gastric lavage may yield positive cultures.
Invasive diagnostic procedures are indicated for patients with suspected extrapulmonary tuberculosis. In addition to specimens of involved sites (e.g., CSF for tuberculous meningitis, pleural fluid and biopsy samples for pleural disease), bone marrow and liver biopsy and culture have a good diagnostic yield in disseminated (miliary) tuberculosis, particularly in HIV-infected patients, who also have a high frequency of positive blood cultures.
In some cases, cultures will be negative, but a clinical diagnosis of tuberculosis will be supported by consistent epidemiologic evidence (e.g., a history of close contact with an infectious patient), a positive PPD skin test, and a compatible clinical and radiographic response to treatment. In the United States and other industrialized countries with low rates of tuberculosis, some patients with limited abnormalities on chest radiographs and sputum positive for AFB are infected with organisms of the M. avium complex or M. kansasii (Chap. 171). Factors favoring the diagnosis of nontuberculous mycobacterial disease over tuberculosis include an absence of risk factors for tuberculosis, a negative PPD skin test, and underlying chronic obstructive pulmonary disease.
Patients with HIV-associated tuberculosis pose several diagnostic problems, as noted above in the description of clinical manifestations. Moreover, HIV-infected patients with sputum culture-positive and AFB-positive tuberculosis may present with a normal chest radiograph. Thus, in a patient with HIV infection, the finding of a normal chest radiograph does not rule out the diagnosis of pulmonary tuberculosis. An additional consideration is that, among relatively severely immunosuppressed AIDS patients in Europe and North America, M. avium complex disease is more common than tuberculosis, usually presenting as a disseminated disease without pulmonary parenchymal involvement.
Adjunctive Diagnostic Tests
A number of methods have been evaluated as adjuncts to standard laboratory diagnosis. The most thoroughly investigated is serologic diagnosis based on detection of antibody to a variety of mycobacterial antigens. However, tests with most of the target antigens have a low predictive value when used in a population with a presumably low probability of disease. Tests aimed at detection of mycobacterial antigen by serologic methods have generally not been sufficiently sensitive to be useful. Nonspecific tests, such as the measurement of adenine deaminase in pleural fluid, have been evaluated but have not gained acceptance.
![]() Treatment
Treatment
Chemotherapy for tuberculosis became possible with the discovery of streptomycin in the mid-1940s. Randomized clinical trials clearly indicated that the administration of streptomycin to patients with chronic tuberculosis reduced mortality and led to cure in the majority of cases. However, monotherapy with streptomycin was frequently associated with the development of resistance to streptomycin and the attendant failure of treatment. With the discovery of para-aminosalicylic acid (PAS) and isoniazid, it became axiomatic that cure of tuberculosis required the concomitant administration of at least two agents to which the organism was susceptible. Furthermore, early clinical trials demonstrated that a long period of treatment-i.e., 12 to 24 months-was required to prevent the recurrence of tuberculosis.
The introduction of rifampin in the early 1970s heralded the era of effective short-course chemotherapy, with a treatment duration of <12 months. The discovery that pyrazinamide, which was first used in the 1950s, augmented the potency of isoniazid/rifampin regimens led to the use of a 6-month course of this triple-drug regimen as standard therapy.
Drugs
Five major drugs are considered the first-line agents for the treatment of tuberculosis: isoniazid, rifampin, pyrazinamide, ethambutol, and streptomycin (Table 169-1). The first four, which are usually given orally, are well absorbed, with peak serum levels at 2 to 4 h and nearly complete elimination within 24 h. These agents are recommended on the basis of their bactericidal activity (ability to rapidly reduce the number of viable organisms), their sterilizing activity (ability to kill all bacilli and thus sterilize the affected organ, measured in terms of the ability to prevent relapses), and their low rate of induction of drug resistance. Rifapentine and rifabutin, two drugs related to rifampin, are also available in the United States. For a detailed discussion of the drugs used for the treatment of tuberculosis, see Chap. 168.
Table 169-1: Dosage Recommendations for Initial Treatment of Tuberculosis in Adults
|
|
|
Dosage |
||
|
Drug |
|
Daily |
|
Thrice Weekly |
|
Isoniazid |
|
5 mg/kg, max. 300 mg |
|
15 mg/kg, max. 900 mg |
|
Rifampin |
|
10 mg/kg, max. 600 mg |
|
10 mg/kg, max. 600 mg |
|
Pyrazinamide |
|
15-30 mg/kg, max. 2 g |
|
50-70 mg/kg, max. 3 g |
|
Ethambutol |
|
15-25 mg/kg |
|
25-30 mg/kg |
|
Streptomycin |
|
15 mg/kg, max. 1 g |
|
25-30 mg/kg, max. 1.5 g |
Because of a lower degree of efficacy and a higher degree of intolerability and toxicity, a number of second-line drugs are used only for the treatment of patients with tuberculosis resistant to first-line drugs. Included in this group are the injectable drugs kanamycin, amikacin, and capreomycin and the oral agents ethionamide, cycloserine, and PAS. Recently, quinolone antibiotics have become the most commonly used second-line drugs. Of available agents, ofloxacin is the most widely used, but levofloxacin and sparfloxacin are the most active, although the latter drug is associated with high rates of photosensitization. Other second-line drugs include clofazimine, amithiozone (thiacetazone, widely used with isoniazid in less wealthy countries but not marketed in North America or Europe), and amoxicillin/clavulanic acid.
Regimens
Short-course regimens are divided into an initial or bactericidal phase and a continuation or sterilizing phase. During the initial phase, the majority of the tubercle bacilli are killed, symptoms resolve, and the patient becomes noninfectious. The continuation phase is required to eliminate semidormant "persisters."
The treatment regimen of choice for virtually all forms of tuberculosis in both adults and children consists of a 2-month initial phase of isoniazid, rifampin, and pyrazinamide followed by a 4-month continuation phase of isoniazid and rifampin (Table 169-2). Except for patients who seem unlikely on epidemiologic grounds to be initially infected with a drug-resistant strain, ethambutol (or streptomycin) should be included in the regimen for the first 2 months or until the results of drug susceptibility testing become available. Treatment may be given daily throughout the course or intermittently (either three times weekly throughout the course or twice weekly following an initial phase of daily therapy). A continuation phase of once-weekly rifapentine and isoniazid appears to be effective for patients who have adhered to the initial-phase treatment and have negative sputum cultures at 2 months. Intermittent treatment is especially useful for patients whose therapy is being directly observed (see below). For patients with sputum culture-negative pulmonary tuberculosis, the duration of treatment may be reduced to a total of 4 months. Pyridoxine (10 to 25 mg/d) should be added to the regimen given to persons at high risk of vitamin deficiency (e.g., alcoholics; malnourished persons; pregnant and lactating women; and patients with conditions such as chronic renal failure, diabetes, and HIV infection or AIDS, which are also associated with neuropathy).
Lack of adherence to treatment regimens is recognized worldwide as the most important impediment to cure. Moreover, the mycobacterial strains infecting patients who do not adhere to the prescribed regimen are especially likely to develop acquired drug resistance. Both patient- and provider-related factors may affect compliance. Patient-related factors include a lack of belief that the illness is significant and/or that treatment will have a beneficial effect; the existence of concomitant medical conditions (notably substance abuse); lack of social support; and poverty, with attendant joblessness and homelessness. Provider-related factors that may promote compliance include the education and encouragement of patients, the offering of convenient clinic hours, and the provision of incentives such as bus tokens.
In addition to specific measures addressing noncompliance, two other strategic approaches are used: direct observation of treatment and provision of drugs in combined formulations. Because it is difficult to predict which patients will adhere to the recommended treatment, all patients should have their therapy directly supervised, especially during the initial phase. In the United States, personnel to supervise therapy are usually available through tuberculosis control programs of local public health departments. Supervision increases the proportion of patients completing treatment and greatly lessens the chances of relapse and acquired drug resistance. Combination products (e.g., isoniazid/rifampin and isoniazid/rifampin/pyrazinamide) are available and are strongly recommended as a means of minimizing the likelihood of prescription error and of the development of drug resistance (as the result of treatment with only one agent). In some formulations of these combination products, the bioavailability of rifampin has been found to be substandard. In North America and Europe, regulatory authorities ensure that combination products are of good quality; however, this type of monitoring cannot be assumed to take place in less affluent countries. Alternative regimens for patients who exhibit drug intolerance or adverse reactions are listed in Table 169-2. However, severe side effects prompting discontinuation of any of the first-line drugs and use of these alternative regimens are uncommon.
Monitoring the Response to Treatment
Bacteriologic evaluation is the preferred method of monitoring the response to treatment for tuberculosis. Patients with pulmonary disease should have their sputum examined monthly until cultures become negative. With the recommended 6-month regimen, more than 80% of patients will have negative sputum cultures at the end of the second month of treatment. By the end of the third month, virtually all patients should be culture-negative. In some patients, especially those with extensive cavitary disease and large numbers of organisms, AFB smear conversion may follow culture conversion. This phenomenon is presumably due to the expectoration and microscopic visualization of dead bacilli. When a patient's sputum cultures remain positive at or beyond 3 months, treatment failure and drug resistance should be suspected (see below). A sputum specimen should be collected at the end of treatment to document cure. If mycobacterial cultures are not practical, then monitoring by AFB smear examination should be undertaken at 2, 5, and 6 months. Smears positive after 5 months are indicative of treatment failure.
Bacteriologic monitoring of patients with extrapulmonary tuberculosis is more difficult and often is not feasible. In these cases, the response to treatment must be assessed clinically.
Monitoring of the response to treatment during chemotherapy by serial chest radiographs is not recommended, as radiographic changes may lag behind bacteriologic response and are not highly sensitive. After the completion of treatment, neither sputum examination nor chest radiography is recommended for follow-up purposes. However, a chest radiograph may be obtained at the end of treatment and used for comparative purposes should the patient develop symptoms of recurrent tuberculosis months or years later. Patients should be instructed to report promptly for medical assessment should they develop any such symptoms.
During treatment, patients should be monitored for drug toxicity (see also Table 168-3). The most common adverse reaction of significance is hepatitis. Patients should be carefully educated about the signs and symptoms of drug-induced hepatitis (e.g., dark urine, loss of appetite) and should be instructed to discontinue treatment promptly and see their health care provider should these symptoms occur. Although biochemical monitoring is not routinely recommended, all adult patients should undergo baseline assessment of liver function (e.g., measurement of levels of hepatic aminotransferases and serum bilirubin). Older patients, those with histories of hepatic disease, and those using alcohol daily should be monitored especially closely (i.e., monthly), with repeated measurements of aminotransferases, during the initial phase of treatment. Up to 20% of patients have small increases in aspartate aminotransferase (up to three times the upper limit of normal) that are accompanied by no symptoms and are of no consequence. For patients with symptomatic hepatitis and those with marked (five- to sixfold) elevations in aspartate aminotransferase, treatment should be stopped and drugs reintroduced one at a time after liver function has returned to normal.
Hypersensitivity reactions usually require the discontinuation of all drugs and rechallenge to determine which agent is the culprit. Because of the variety of regimens available, it is usually not necessary-although it is possible-to desensitize patients. Hyperuricemia and arthralgia caused by pyrazinamide can usually be managed by the administration of acetylsalicylic acid; however, pyrazinamide treatment should be stopped if the patient develops gouty arthritis. Individuals who develop autoimmune thrombocytopenia secondary to rifampin therapy should not receive the drug thereafter. Similarly, the occurrence of optic neuritis with ethambutol and the development of eighth-nerve damage with streptomycin are indications for permanent discontinuation of these respective drugs. Other common manifestations of drug intolerance, such as pruritus and gastrointestinal upset, can generally be managed without the interruption of therapy.
Treatment Failure and Relapse
As stated above, treatment failure should be suspected when a patient's sputum cultures remain positive after 3 months or when AFB smears remain positive after 5 months. In the management of such patients, it is imperative that the current isolate be tested for susceptibility to first- and second-line agents. When the results of susceptibility testing are expected to become available within a few weeks, changes in the regimen can be postponed until that time. However, if the patient's clinical condition is deteriorating, an earlier change in regimen may be indicated. A cardinal rule in the latter situation is always to add more than one drug at a time to a failing regimen: at least two and preferably three drugs that have never been used should be added. The patient may continue to take isoniazid and rifampin along with these new agents pending the results of susceptibility tests.
The mycobacterial strains infecting patients who experience a relapse after apparently successful treatment are less likely to have acquired drug resistance (see below) than are strains from patients in whom treatment has failed. However, if the regimen administered initially does not contain rifampin (and thus is not a short-course regimen), the probability of isoniazid resistance is high. Acquired resistance is uncommon among strains from patients who relapse after completing a short course of therapy. However, it is prudent to begin the treatment of all relapses with all five first-line drugs pending the results of susceptibility testing. In less affluent countries and other settings where facilities for culture and drug susceptibility testing are not available, a standard regimen should be used in all instances of relapse and treatment failure (Table 169-2).
Adjunctive Glucocorticoid Therapy
The use of glucocorticoids for adjunctive treatment of tuberculosis is justified by their potent anti-inflammatory activity in a disease where host response plays a major role. There is a sound basis for using glucocorticoids in tuberculous meningitis and pericarditis to hasten clinical improvement (see above). Long-term benefits are limited to reduced mortality in effusive-constrictive pericarditis and decreased neurologic sequelae in meningitis. Studies have indicated that the usefulness of these agents in pleuritis may be less important than previously thought. There is not yet conclusive evidence that glucocorticoids produce benefits in patients with acute life-threatening pulmonary tuberculosis, including ARDS. In general, depending upon the urgency and severity of clinical conditions, prednisone may be administered at a daily dose of 20 to 60 mg for up to 6 weeks. In meningitis, dexamethasone (up to 12 mg/d) is the preferred drug; therapy continues for 4 to 6 weeks, with gradual tapering of the dose after the first 2 weeks. Rifampin interaction with glucocorticoids, resulting in accelerated metabolism and potential adrenal crisis, must be taken into account. Caution should be exercised in the use of glucocorticoids in HIV-infected patients.
Drug-Resistant Tuberculosis
Strains of M. tuberculosis resistant to individual drugs arise by spontaneous point mutations in the mycobacterial genome, which occur at low but predictable rates. Because there is no cross-resistance among the commonly used drugs, the probability that a strain will be resistant to two drugs is the product of the probabilities of resistance to each drug and thus is low. The development of drug-resistant tuberculosis is invariably the result of monotherapy-i.e., the failure of the health care provider to prescribe at least two drugs to which tubercle bacilli are susceptible or of the patient to take properly prescribed therapy.
Drug-resistant tuberculosis may be either primary or acquired. Primary drug resistance is that in a strain infecting a patient who has not previously been treated. Acquired resistance develops during treatment with an inappropriate regimen. In North America and Europe, rates of primary resistance are generally low, and isoniazid resistance is most common. In the United States, while isoniazid resistance was stable at about 8% in 1993 through 1996, rates of MDR tuberculosis were declining. Resistance rates are higher among foreign-born and HIV-infected patients. Worldwide, MDR tuberculosis is a serious problem in some regions, especially in the former USSR and parts of Asia. As noted above, drug-resistant tuberculosis can be prevented by adherence to the principles of sound therapy: the inclusion of at least two bactericidal drugs to which the organism is susceptible (in practice, four drugs are commonly given in the initial phase) and the verification that patients complete the prescribed course.
Although the 6-month regimen described in Table 169-2 is highly effective for patients with initial isoniazid-resistant disease, it is prudent to extend treatment to 9 months and to include ethambutol throughout. Alternatively, rifampin, pyrazinamide, and ethambutol may be given for 6 months. For disease with high-level isoniazid resistance, isoniazid probably does not contribute to a successful outcome and can be omitted. MDR tuberculosis is more difficult to manage than is disease caused by a drug-susceptible organism, especially because resistance to other first-line drugs as well as to isoniazid and rifampin is common. For strains resistant to isoniazid and rifampin, combinations of ethambutol, pyrazinamide, and streptomycin (or, for those resistant to streptomycin as well, another injectable agent such as amikacin), given for 12 to 18 months in all and for at least 9 months after sputum culture conversion, may be effective. Many authorities would add a quinolone antibiotic to this regimen. For patients with bacilli resistant to all of the first-line agents, cure may be attained with a combination of four second-line drugs, including one injectable agent (Table 169-2). The optimal duration of treatment in this situation is not known; however, a duration of up to 24 months is recommended. For patients with localized disease and sufficient pulmonary reserve, lobectomy or pneumonectomy may be helpful. Because the management of patients with MDR tuberculosis is complicated by both social and medical factors, care of these patients should be restricted to specialists and tuberculosis control programs.
Special Clinical Situations
Although comparative clinical trials of treatment for extrapulmonary tuberculosis are limited, the available evidence indicates that most forms of disease can be treated with the 6-month regimen recommended for patients with pulmonary disease. The American Academy of Pediatrics recommends that children with bone and joint tuberculosis, tuberculous meningitis, or miliary tuberculosis receive a minimum of 12 months of treatment.
Treatment for tuberculosis may be complicated by underlying medical problems that require special consideration (see also Table 168-1). As a rule, patients with chronic renal failure should not receive aminoglycosides and should receive ethambutol only if serum levels can be monitored. Isoniazid, rifampin, and pyrazinamide may be given in the usual doses in cases of mild to moderate renal failure, but the dosages of isoniazid and pyrazinamide should be reduced for all patients with severe renal failure except those undergoing hemodialysis. Patients with hepatic disease pose a special problem because of the hepatotoxicity of isoniazid, rifampin, and pyrazinamide. Patients with severe hepatic disease may be treated with ethambutol and streptomycin and, if required, with isoniazid and rifampin under close supervision. The use of pyrazinamide by patients with liver failure should be avoided. Silicotuberculosis necessitates the extension of therapy by at least 2 months. Patients with HIV infection or AIDS appear to respond well to standard 6-month therapy, although treatment may need to be prolonged if the response is suboptimal. Rifampin, a powerful inducer of hepatic microsomal enzymes, shortens the half-life of HIV protease inhibitors and therefore is contraindicated for patients receiving these drugs; instead, these individuals should be given rifabutin (150 mg/d or 300 mg twice weekly) with either indinavir or nelfinavir. Studies have shown that total systemic drug exposure, especially for rifampin, is reduced in HIV-infected patients because of decreased bioavailability secondary to malabsorption. The clinical importance of this phenomenon remains unclear.
The regimen of choice for pregnant women (see also Table 168-1) is 9 months of treatment with isoniazid and rifampin supplemented by ethambutol for the first 2 months. When required, pyrazinamide may be given, although there are no data concerning its safety in pregnancy. Streptomycin is contraindicated because it is known to cause eighth-cranial-nerve damage in the fetus. Treatment for tuberculosis is not a contraindication to breast feeding; most of the drugs administered will be present in small quantities in breast milk, albeit at concentrations far too low to provide any therapeutic or prophylactic benefit to the child.
Prevention
By far the best way to prevent tuberculosis is to diagnose infectious cases rapidly and administer appropriate treatment until cure. Additional strategies include BCG vaccination and preventive chemotherapy.
BCG Vaccination
BCG was derived from an attenuated strain of M. bovis and was first administered to humans in 1921. Many BCG vaccines are available worldwide; all are derived from the original strain, but the vaccines vary in efficacy. In fact, estimates of efficacy from randomized, placebo-controlled trials have ranged from 80% to nil. A similar range of efficacy was found in recent observational studies (case-control, historic cohort, and cross-sectional) in areas where infants are vaccinated at birth. These studies also found higher rates of efficacy in the protection of infants and young children from relatively serious forms of tuberculosis, such as tuberculous meningitis and miliary tuberculosis.
BCG vaccine is safe and rarely causes serious complications. The local tissue response begins 2 to 3 weeks after vaccination, with scar formation and healing within 3 months. Side effects-most commonly, ulceration at the vaccination site and regional lymphadenitis-occur in 1 to 10% of vaccinated persons. Some vaccine strains have caused osteomyelitis in approximately one case per million doses administered. Disseminated BCG infection and death have occurred in 1 to 10 cases per 10 million doses administered, although this problem is restricted almost exclusively to persons with impaired immunity, such as children with severe combined immunodeficiency syndrome (SCIDS) or adults with HIV infection. BCG vaccination induces PPD reactivity, which tends to wane with time. The presence or size of PPD skin-test reactions after vaccination does not predict the degree of protection afforded.
BCG vaccine is recommended for routine use at birth in countries with high tuberculosis prevalence. However, because of the low risk of transmission of tuberculosis in the United States and the unreliable protection afforded by BCG, the vaccine has never been recommended for general use in the United States. Currently, vaccination should be considered for PPD-negative infants and children who reside in settings where the likelihood of M. tuberculosis transmission and subsequent infection is high, provided no other measures can be implemented (e.g., removing the child from the source of infection). BCG vaccination may also be considered for health care workers who are employed in settings where the risk of infection by MDR strains is high despite implementation of comprehensive tuberculosis control measures. The CDC has recommended that HIV-infected adults and children not receive BCG vaccine, although the WHO has recommended that asymptomatic HIV-infected children residing in tuberculosis-endemic areas receive BCG.
Treatment of Latent Tuberculosis Infection
A major component of tuberculosis control in the United States is the treatment of selected persons with latent tuberculosis infection to prevent active disease. This intervention (formerly called preventive chemotherapy or chemoprophylaxis) is based on the results of a large number of randomized, placebo-controlled clinical trials demonstrating that a 6- to 12-month course of isoniazid reduces the risk of active tuberculosis in infected people by ![]() 90%. Analysis of available data indicates that the optimal duration of treatment is 9 to 10 months. In the absence of reinfection, the protective effect is believed to be lifelong. Clinical trials have also shown that isoniazid reduces rates of tuberculosis among PPD-positive persons with HIV infection. Studies in HIV-infected patients have demonstrated the effectiveness of a shorter course of rifampin-based treatment.
90%. Analysis of available data indicates that the optimal duration of treatment is 9 to 10 months. In the absence of reinfection, the protective effect is believed to be lifelong. Clinical trials have also shown that isoniazid reduces rates of tuberculosis among PPD-positive persons with HIV infection. Studies in HIV-infected patients have demonstrated the effectiveness of a shorter course of rifampin-based treatment.
In most cases, candidates for treatment of latent tuberculosis (Table 169-3) are identified by PPD skin testing of persons in defined high-risk groups. For skin testing, 5 tuberculin units of polysorbate-stabilized PPD should be injected intradermally into the volar surface of the forearm (Mantoux method). Multipuncture tests, which may be useful for screening large populations, are not recommended for this purpose; any positive reaction to a multipuncture test must be confirmed by Mantoux testing. Reactions are read at 48 to 72 h as the transverse diameter in millimeters of induration; the diameter of erythema is not considered. In some persons, PPD reactivity wanes with time but can be recalled by a second skin test administered 1 week or more after the first (i.e., two-step testing). For persons undergoing periodic PPD skin testing, such as health care workers and individuals admitted to long-term-care institutions, initial two-step testing may preclude subsequent misclassification of persons with boosted reactions as PPD converters.
Table 169-3: Tuberculin Reaction Size and Treatment of Latent Tuberculosis Infection
|
Risk Group Tuberculin |
Reaction, mm |
|
HIV-infected |
> 5 |
|
Close contacts of tuberculosis patients |
>5 |
|
Persons with fibrotic lesions on chest radiography |
>5 |
|
Recently infected persons (<2 years ) |
>10 |
|
Persons with high-risk medical conditions |
>10 |
|
High-risk group, <35 years of age |
>10 |
|
Low-risk group, <35 years of age |
>15 |
The cutoff for a positive skin test (and thus for treatment) is related both to the probability that the reaction represents true infection and to the likelihood that the individual, if truly infected, will develop tuberculosis (Table 169-3). Thus positive reactions for close contacts of infectious cases, persons with HIV infection, and previously untreated persons whose chest radiograph is consistent with healed tuberculosis are defined as an area of induration 5 mm in diameter. A 10-mm cutoff is used to define positive reactions in most other at-risk persons. For persons with a very low risk of developing tuberculosis if infected, a cutoff of 15 mm is used. Persons with a history of BCG vaccination may receive treatment, especially if BCG was given many years before.
Some PPD-negative individuals are also candidates for treatment. Infants and children who have come into contact with infectious cases should be treated and should have a repeat skin test 2 or 3 months after contact ends. Those whose test results remain negative should discontinue treatment. HIV-infected persons who have been exposed to an infectious tuberculosis patient should receive treatment regardless of the PPD test result.
Isoniazid is administered at a daily dose of 5 mg/kg (up to 300 mg/d) for 9 months. On the basis of cost-benefit analyses, a 6-month period of treatment has been recommended in the past and may be considered for HIV-negative adults with normal chest radiographs when financial considerations are important. When supervised treatment is desirable and feasible, isoniazid may be given at a dose of 15 mg/kg (up to 900 mg) twice weekly. There are two recommended alternative regimens for adults: 2 months of daily rifampin plus pyrazinamide and 4 months of daily rifampin. Although the 2-month regimen may be associated with increased drug intolerance, it may be useful in situations where long courses of isoniazid have not been feasible (e.g., jails). Either regimen should be considered for persons who are likely to have been infected with an isoniazid-resistant strain.
Contraindications to treatment with isoniazid and pyrazinamide include active liver disease. Since the major adverse reaction to these drugs is hepatitis, persons at increased risk of toxicity (e.g., those abusing alcohol daily and those with a history of liver disease) should undergo baseline and then monthly assessment of liver function during treatment. All patients should be carefully educated about hepatitis and instructed to discontinue use of the drug immediately should any symptoms develop. Moreover, patients should be seen and questioned monthly during therapy about adverse reactions and should be given no more than 1 month's supply of drug at each visit.
It may be more difficult to ensure compliance when treating persons with latent infection than when treating those with active tuberculosis. If family members of active cases are being treated, compliance and monitoring may be easier. When feasible, twice-weekly supervised therapy may increase the likelihood of completion. As in active cases, the provision of incentives may also be helpful.
Basics of Control
The highest priority in any tuberculosis control program is the prompt detection of cases and the provision of directly observed short-course chemotherapy to all tuberculosis patients, with emphasis on the cure of sputum smear-positive cases. In addition, in low-prevalence countries with adequate resources, screening of high-risk groups (such as immigrants from high-prevalence countries and HIV-seropositive persons) is recommended. Identification of active cases of tuberculosis should be followed by treatment. PPD-positive high-risk persons should be treated for latent infection. Contact investigation is an important component of efficient tuberculosis control. In the United States, a great deal of attention has been given to the transmission of tuberculosis (particularly in association with HIV infection) in institutional settings such as hospitals, homeless shelters, and prisons. Measures to limit such transmission include respiratory isolation of persons with suspected tuberculosis until they are proven to be noninfectious (i.e., by sputum AFB smear negativity), proper ventilation in rooms of patients with infectious tuberculosis, use of ultraviolet lights in areas of increased risk of tuberculosis transmission, and periodic screening of personnel who may come into contact with known or unsuspected cases of tuberculosis. In the past, radiographic surveys, especially those conducted with portable equipment and miniature films, were advocated for case finding. Today, however, the prevalence of tuberculosis in industrialized countries is sufficiently low that "mass miniature radiography" is not cost-effective. As mentioned above, current recommendations for the prevention and treatment of tuberculosis in HIV-infected individuals have been published by the CDC.
In high-prevalence countries, tuberculosis control programs should be based on the following key elements: (1) case detection through microscopic examination of sputum from patients who present to health care facilities with cough of >3 weeks' duration; (2) administration of standard short-course chemotherapy to all sputum smear-positive patients, with direct observation of drug ingestion; (3) establishment and maintenance of a system of regular drug supply; and (4) establishment and maintenance of an effective surveillance and treatment-monitoring system allowing an analysis of treatment outcomes (e.g., cure, completion of treatment without bacteriologic proof of cure, death, treatment failure, and default) in all cases registered.